In this issue of Blood, Wölfler and colleagues describe a mouse model that allows tracking of hematopoietic cells that express C/EBPα and the progeny thereof. Their data indicate that expression of C/EBPα drives commitment of multipotent progenitors toward a myeloid cell fate, but lymphoid potential is retained as well.
Multipotent hematopoietic stem cells (HSCs) that reside in the bone marrow can give rise to all myeloid and lymphoid lineages of the blood, but the process of lineage specification is complex. Cytokines and growth factors are critically important, although there is still lively debate about the “instructive” versus “permissive” nature of their actions (see comment in Enver and Jacobsen1 ). Cytokines are able to instruct progenitor cells to develop into a certain lineage,2 but stem cell–intrinsic programs that predetermine lineage fate appear to exist as well (reviewed in Schroeder3 ). Both these extrinsic and intrinsic cues will ultimately lead to changes in gene expression programs that will guide the differentiation of progenitors into mature cell types, and the activation of transcription factors is key to these processes.
One such transcription factor is the CCAAT/enhancer-binding protein α (C/EBPα). It has been demonstrated that C/EBPα is essential for the formation of granulocytes and in particular for the transition from the common myeloid progenitor (CMP) toward the granulocyte/monocyte progenitor (GMP).4 Initial studies suggested that C/EBPα is expressed at low levels in HSCs, is up-regulated upon maturation toward CMPs and GMPs, and is not expressed in erythroid/megakaryocyte progenitors (MEPs) or lymphoid cells.5 However, little was known about the precise cell type in which C/EBPα is first expressed within the hematopoietic hierarchy, and what the ultimate fate of the progeny of these C/EBPα-expressing cells would be.
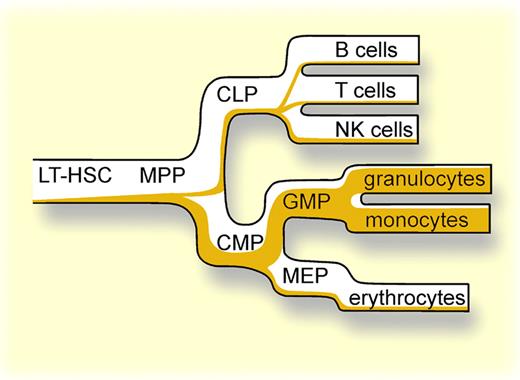
Wölfler and colleagues make use of a model in which Cre recombinase is knocked-in in the Cebpa locus and Cre is therefore expressed under the control of the endogenous Cebpa promoter.6 These mice lack one Cebpa allele but this does not impair myeloid development or steady-state hematopoiesis. Next, these Cebpa+/Cre mice were crossed with ROSA26 EYFP reporter mice, in which EYFP is only expressed after Cre-mediated recombination of loxP sites within the locus. Thus, as soon as the Cebpa promoter becomes activated, expression of Cre will allow the expression of EYFP. Not only will cells in which the Cebpa promoter is activated become EYFP+, but also all progeny of these cells will be positive for EYFP since the loxP sites are irreversibly deleted, regardless of whether C/EBPα remains expressed (see figure).
In each hematopoietic compartment, the relative amount of cells that express C/EBPα or the progeny thereof is indicated in orange.
In each hematopoietic compartment, the relative amount of cells that express C/EBPα or the progeny thereof is indicated in orange.
Based on this mouse model several conclusions can be drawn: (1) only 4% of the most immature stem cells express C/EBPα, and the number of cells that express (or have expressed) C/EBPα increases to approximately 15% in multipotent progenitors; (2) upon differentiation along the myeloid lineage from CMP to GMPs an increasing number of cells express (or have expressed) C/EBPα, and practically all mature monocytes and granulocytes have expressed C/EBPα at least at some point during their development; and (3) despite the notion that erythroid and lymphoid cells do not express C/EBPα, it is clear from these tracing studies that at least some of these cells did express C/EBPα early in their development. In particular, this last point raises some important issues because it argues against a lineage-restrictive role for C/EBPα. Apparently, the expression of C/EBPα in immature progenitors does not prevent the differentiation toward a lymphoid or erythroid cell fate, even though the majority of C/EBPα-EYFP+ progenitor cells become myeloid cells. These findings are in line with the notion that the promiscuous expression of myeloid, erythroid, and lymphoid genes precedes the actual lineage commitment.7,8
The Cebpa+/CreR26EYFP mouse model allows the identification of C/EBPα-expressing cells and particularly their progeny in an in vivo setting, but does not allow the quantification of C/EBPα expression at the protein level, which would for instance be possible in a transgenic mouse in which EYFP would be directly fused to C/EBPα. In addition, this C/EBPα-EYFP model would provide more insight into the kinetics of C/EBPα expression, and would allow the analysis of exact down-modulation of C/EBPα upon commitment along the lymphoid or erythroid lineage. Since the dosage and timing most likely matter, it will be interesting to develop those models as well.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■