Abstract
An estimated 6% to 7% of the earth's population carries a mutation affecting red blood cell function. The β-thalassemias and sickle cell disease are the most common monogenic disorders caused by these mutations. Increased levels of γ-globin ameliorate the severity of these diseases because fetal hemoglobin (HbF; α2γ2) can effectively replace adult hemoglobin (HbA; α2β2) and counteract polymerization of sickle hemoglobin (HbS; α2βS2). Therefore, understanding the molecular mechanism of globin switching is of biologic and clinical importance. Here, we show that the recently identified chromatin factor Friend of Prmt1 (FOP) is a critical modulator of γ-globin gene expression. Knockdown of FOP in adult erythroid progenitors strongly induces HbF. Importantly, γ-globin expression can be elevated in cells from β-thalassemic patients by reducing FOP levels. These observations identify FOP as a novel therapeutic target in β-hemoglobinopathies.
Introduction
In humans, the composition of the hemoglobin tetramer changes several times during development. The final “switch” occurs around birth, when fetal hemoglobin (HbF), containing 2 α-globin and 2 γ-globin polypeptides, is replaced by adult hemoglobin (HbA), containing 2 α-globin and 2 β-globin polypeptides.1 In the large majority of healthy adults, HbF contributes approximately 1% to total Hb. In contrast, higher levels of HbF are observed in subpopulations of β-thalassemia and sickle cell anemia patients and can significantly alleviate the disease phenotype.2 The molecular mechanism of globin switching and the variation in adult γ-globin repression are not well understood but most probably involve developmental stage-specific changes in transcription factors and/or chromatin remodeling complexes. Indeed, fetal- and adult-specific splice variants of the repressor BCL11A have recently been shown to have a crucial role in proper γ-globin regulation.3 Genome-wide association studies indicate that common single nucleotide polymorphisms in the BCL11A, HSB1L-MYB, and HBB loci account for less than or equal to 50% of the variation in HbF levels, suggesting that additional factors are involved.4-8 Here, we show that the recently identified chromatin factor Friend of Prmt1 (FOP) plays a critical role in fetal globin expression.
Methods
Cells
Mouse erythroid progenitor cultures were derived from fetal livers of embryonic day 12.5 PAC8 embryos9 and expanded as described previously.10 Human erythroid progenitors were cultured from buffy coats in serum-free medium as described previously.11 β-Thalassemia patients were transfusion-dependent, nonresponders to hydroxyurea treatment and negative for the XmnI polymorphism. Patient 4 was positive for β-globin gene defect IVSI-5/IVSI-5; patient 9 was positive for C39/C39. These studies were approved by the Institutional Ethical Review Board of Erasmus MC.
RNA analysis
Polymerase chain reaction (PCR) analysis was performed with Platinum Taq (Invitrogen). Primers used are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Quantitative S1 nuclease protection assays were performed as described.12
Lentivirus-mediated knockdown
Clones from The RNAi Consortium13 were used for knockdown experiments in mouse cells, including control (SHC002) and Fop (TRCN0000182232). For knockdown experiments in human cells, a short hairpin against FOP (GGAGCAGCTGGACAACCAA; target sequence mutated to GGAGCAACTAGATAACCAA in the rescue cDNA) and a control sequence (GACTCCAGTGGTAATCTAC) were cloned into a modified pRRLsin.sPPT.CMV.GFP.Wpre lentiviral vector.14 Lentivirus was produced by transient transfection of 293T cells according to standard protocols.15
Western blotting and immunohistochemistry
Western blot analysis was performed as described.16 Nitrocellulose membranes were blocked in 1% bovine serum albumin, incubated with appropriate antibodies, and analyzed/quantified using the Odyssey Infrared Imaging System (Li-Cor Biosciences). The following primary antibodies were used: FOP (KT64) from Absea Biotechnology, SOX6 (NBP1-19537) from Novus, and actin (sc-1616), BCL11A (sc-56013), and hemoglobin-γ (sc-21756) from Santa Cruz Biotechnology. For immunohistochemistry, cells were spotted on poly-prep slides (Sigma-Aldrich), fixed with 4% paraformaldehyde, permeabilized in 10mM citric acid (pH 6.0), and blocked with 5% bovine serum albumin. Primary antibody incubation was performed in blocking solution for 16 hours at 4°C, followed by peroxidase staining.
Results and discussion
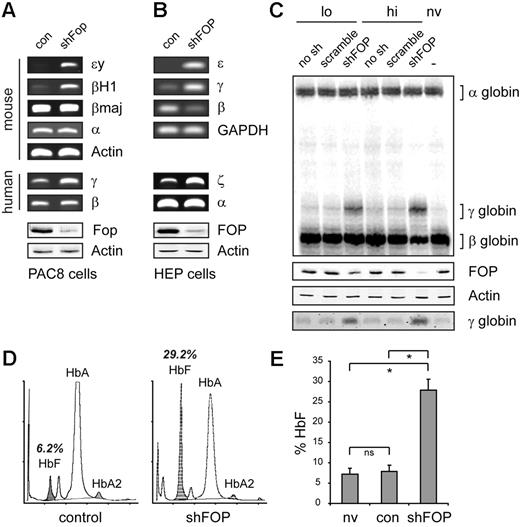
We recently identified FOP, also known as small protein rich in arginine and glycine, encoded by the human C1orf77 and mouse 2500003M10Rik genes, respectively, as a chromatin-associated protein with a critical role in transcriptional regulation, including estrogen-dependent gene induction in breast cancer cells.17,18 To study whether Fop had a role in the expression of globin genes, we used growth factor-dependent, differentiation-competent cultures of mouse fetal liver (FL) cells containing a transgenic single-copy integration of the entire human β-globin locus. This allowed study of the expression of both mouse and human globin genes. Lentiviral-mediated knockdown of Fop expression resulted in more than 80% reduction of Fop protein 7 days after transduction (Figure 1A). PCR analysis shows the specific up-regulation of the mouse ϵy and βH1 genes, as well as the human γ-globin gene in Fop-depleted cells (Figure 1A). No changes were observed in the levels of β-actin, α-globin, and βmaj transcripts, indicating that overall differentiation was not affected.
Fop regulates embryonic globin genes in mouse and human erythroid cells. Lentiviral-mediated knockdown of Fop in (A) mouse erythroid progenitor cells containing a single copy of the human globin locus (PAC8) and (B) primary HEP show the specific induction of mouse and human fetal β-like globin genes, as demonstrated by qualitative PCR analysis. Western blot analysis was used to validate Fop knockdown levels (bottom panels). (C) Quantitative S1 nuclease protection assay and Western blot analysis demonstrate significant γ-globin reactivation in HEP cells with reduced FOP expression. (D) Globin high-performance liquid chromatography of transduced HEP cells after 11 days of culture. Peaks for HbA (α2β2), HbA2 (α2δ2), and HbF (α2γ2) are indicated. (E) The shRNA-mediated knockdown of FOP increases HbF levels on average approximately 3.7 times in cultured human erythroid progenitor cells. Cultures from 3 independent healthy donors were analyzed. ns indicates not significant. *Significant difference (Mann-Whitney test, P < .05).
Fop regulates embryonic globin genes in mouse and human erythroid cells. Lentiviral-mediated knockdown of Fop in (A) mouse erythroid progenitor cells containing a single copy of the human globin locus (PAC8) and (B) primary HEP show the specific induction of mouse and human fetal β-like globin genes, as demonstrated by qualitative PCR analysis. Western blot analysis was used to validate Fop knockdown levels (bottom panels). (C) Quantitative S1 nuclease protection assay and Western blot analysis demonstrate significant γ-globin reactivation in HEP cells with reduced FOP expression. (D) Globin high-performance liquid chromatography of transduced HEP cells after 11 days of culture. Peaks for HbA (α2β2), HbA2 (α2δ2), and HbF (α2γ2) are indicated. (E) The shRNA-mediated knockdown of FOP increases HbF levels on average approximately 3.7 times in cultured human erythroid progenitor cells. Cultures from 3 independent healthy donors were analyzed. ns indicates not significant. *Significant difference (Mann-Whitney test, P < .05).
Next, we studied the role of FOP on globin expression in human erythroid progenitor (HEP) cells cultured from adult peripheral blood (PB). To deplete FOP in human cells, we used a second lentiviral construct expressing a different shRNA sequence and green fluorescent protein as a marker. Eight days after transduction, more than 97% of the cells were positive for green fluorescent protein (not shown), and the FOP protein level was reduced by more than 80% (Figure 1B). Cells were grown for 3 more days in medium supporting differentiation and analyzed. We observed a marked increase in the ϵ- and γ-globin transcripts, whereas the level of β-globin mRNA declined, suggesting a competitive advantage for the expression of the embryonic/fetal β-like globin genes when FOP expression is reduced (Figure 1B). Furthermore, ζ-globin, the embryonic gene within the α-globin cluster, was also reactivated in these experiments (Figure 1B). Although the embryonic ζ- and ϵ-globins were clearly induced in the FOP-depleted cells, their contribution to total globin mRNA output was less than 1%, as calculated by quantitative PCR (not shown). We therefore focused on γ- and β-globin regulation. To directly measure the ratio of γ- and β-globin transcripts, we used quantitative S1 nuclease protection assays. This revealed a dose-dependent induction of γ-globin, varying from 15% of total β-like globin in cells with a partial knockdown of FOP, to 31% in cells in which FOP was almost completely depleted (Figure 1C). In cells transduced with lentivirus expressing no or a nontargeting shRNA, the γ-globin level did not change compared with nontransduced cells (Figure 1C). Lentiviral knockdown of p53 had no influence on γ-globin expression, demonstrating that elevated γ-globin levels are not the result of activating the RNAi machinery per se (not shown). Furthermore, the wild-type phenotype was restored by coexpression of an isocoding but unmatched FOP mRNA (supplemental Figure 2).
Next, we tested whether the induction of γ-globin mRNA was accompanied by elevated levels of HbF using high-performance liquid chromatography. In the FOP knockdown cells, HbF contributed significantly to the amount of total Hb (Figure 1D-E).
Immunohistochemistry showed that, although to a varying degree, all cells express γ-globin, indicating that the elevated HbF level is not the result of a minor responsive cell population (Figure 2A).
Reduced FOP expression correlates with high HbF in primary human cells. (A) Immunohistochemistry for γ-globin shows that more than 90% of FOP knockdown cells contribute to elevated HbF levels. (B) PB HEP cells express FOP at approximately 2.5 times higher level than FL cells. The level of FOP protein relative to actin; 3 FL and 3 PB donors were analyzed. *Significant difference (Mann-Whitney test, P < .05). (C) Reduced levels of FOP lower the expression of SOX6 but do not change the expression of BCL11A. (D) Quantitative S1 nuclease protection assay demonstrates an approximately 50% increase of γ-globin expression in erythroid progenitors from β-thalassemic patients when FOP levels are reduced.
Reduced FOP expression correlates with high HbF in primary human cells. (A) Immunohistochemistry for γ-globin shows that more than 90% of FOP knockdown cells contribute to elevated HbF levels. (B) PB HEP cells express FOP at approximately 2.5 times higher level than FL cells. The level of FOP protein relative to actin; 3 FL and 3 PB donors were analyzed. *Significant difference (Mann-Whitney test, P < .05). (C) Reduced levels of FOP lower the expression of SOX6 but do not change the expression of BCL11A. (D) Quantitative S1 nuclease protection assay demonstrates an approximately 50% increase of γ-globin expression in erythroid progenitors from β-thalassemic patients when FOP levels are reduced.
Next, we examined the level of FOP protein in FL and PB-derived HEP cells in multiple samples (supplemental Figure 3). As shown in Figure 2B, FL cells express approximately 2.5 times less FOP than PB cells, suggesting that modulation of FOP expression might be involved in globin switching during development.
It is possible that the elevated γ-globin expression by the FOP knockdown is mediated via BCL11A, the only known potent repressor of the γ-globin genes, or SOX6, a cofactor of BCL11A in γ-globin silencing.19 We therefore tested whether the FOP knockdown changes the expression of BCL11A and SOX6. The results show that BCL11A levels are unaffected when γ-globin is induced in FOP-depleted cells (Figure 2C). In contrast, expression of SOX6 is reduced, indicating that depletion of FOP might modulate SOX6-dependent silencing of γ globin in adult HEP cells.
Next, we tested whether reduction of FOP expression resulted in elevated γ-globin expression in erythroid progenitors from β-thalassemic patients. Although these cells already expressed relatively high levels of HbF, γ-globin expression was doubled after FOP depletion in both β-thalassemic samples tested (Figure 2D). This indicates that interference with FOP activity could be an effective approach to increase HbF in β-hemoglobinopathy patients. Intriguingly, FOP is a target of methylation by the PRMT1 and PRMT5 arginine methyltransferases.17 These enzymes are thought to regulate globin expression by dictating histone modifications.20-22 Although it is currently unclear how FOP regulates γ-globin expression, we suggest that, in addition to histones, it is a crucial PRMT target for globin gene regulation. Collectively, the results reported here identify FOP as a novel potential therapeutic target in β-globin-related disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rianne Verzijl, Arjan van den Berg, Teus van Gent, Petros Papadopoulos, and Ali Aghajanirefah for technical assistance and Dr Anita Rijneveld for support.
This work was supported by The Netherlands Genomics Initiative (93518009), Erasmus MC (MRace; 296088), the Landsteiner Foundation for Blood Transfusion Research (0615), The Netherlands Scientific Organization (DN 82-294), the Center for Biomedical Genetics, the European Union FP6 EUtracc consortium, and Division of Extramural Research Activities, National Heart, Lung, and Blood Institute (grant 5 RO1 HL073455-04).
National Institutes of Health
Authorship
Contribution: T.B.v.D., N.G., and F.P. performed the experiments; T.B.v.D., F.G., and S.P. designed the research; K.v.L. and M.v.L. provided vital analytical tools; and T.B.v.D. and S.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thamar van Dijk or Sjaak Philipsen, Erasmus MC, Department of Cell Biology, Room Ee720a, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands; e-mail: t.vandijk@erasmusmc.nl or j.philipsen@erasmusmc.nl.