Abstract
Abstract 1015
Natural killer (NK) cells are a component of the innate immune system that target both tumors and virally infected cells. NK cell killing of tumors is regulated by a delicate balance of activating and inhibitory receptors. These inhibitory receptors bind HLA ligands which prevent NK cell targeting of normal “self” cells. Up regulation of surface expression of HLA molecules has been utilized by tumors as a method to evade NK cell cytotoxicity. Disrupting the function or expression of inhibitory receptors on NK cells could potentially be used as a method to overcome this effect. While most inhibitory receptors are present in only a subset of NK cells, NK cells universally express the HLA-E binding inhibitory receptor NKG2A. We hypothesized that siRNA inactivation of NK cell NKG2A would could be used as a therapeutic approach to enhance NK cell tumor cytotoxicity in vivo.
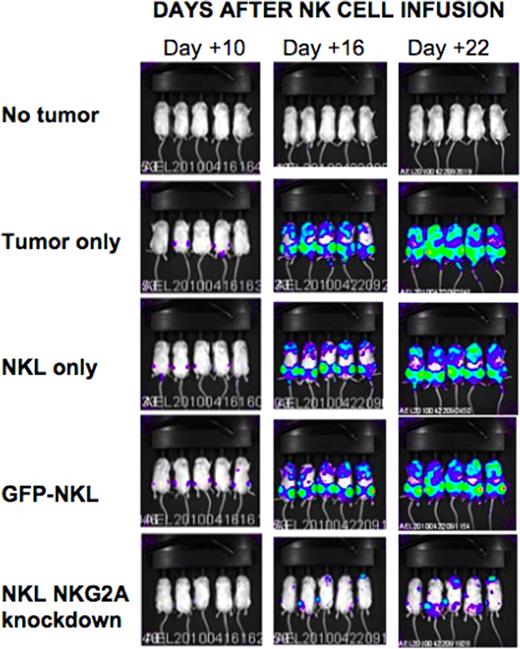
The human natural killer cell line NKL was transduced with lentiviral vectors encoding shRNA targeting various regions of the NKG2A transcript. Following lentiviral transduction, knockdown of receptor expression was confirmed by flow cytometry and RT-qPCR. Compared to wild type (WT) and GFP-transduced NKL controls, NKG2A silenced NKL cells had increased secretion of IFN-gamma and Fas-L by ELISA and increased granzymes A and B and Nkp30 expression by flow cytometry. In contrast, expression of NKG2D, Nkp44, Nkp46, LFA-1, DNAM, and TRAIL was not altered by NKG2A silencing. Chromium-based cytotoxicity assays showed shRNA knockdown of NKG2A significantly enhanced NK cell cytotoxicity of tumor cells: at a 20:1 effector to target ratio, NKG2A knockdown NKLs, WT NKLs and GFP-transduced NKLs induced 68.9%, 8.2% and 8.3% lysis respectively of 721.221 EBV-LCL tumor targets (p=0.001). Remarkably, NKG2A silencing enhanced NKL killing of both HLA-E positive (721.221 EBV-LCL and 526 melanoma cells) and HLA-E negative (K562) tumor cell lines, suggesting NKG2A inactivation increased NK cell cytotoxicity through both HLA-E dependent and independent mechanisms. Using a xenogeneic model, we next explored the in vivo effects of transferring NKG2A silenced NK cells in tumor bearing mice. Immunodeficient NSG mice were injected with 1 million human luciferase transduced 721.221 HLA-E expressing EBV-LCL tumor cells. Twenty-four hours later, tumor-bearing mice were injected with 2–5 million WT NKL cells, GFP-control-transduced NKL, or NKG2A silenced NKL cells, then received IL-2 sq for 10 days to induce in vivo NK cell proliferation. NKL numbers in blood were subsequently analyzed by flow cytometry and tumor burden was assessed by luciferase-based bioluminescence imaging (BLI). At 16 and 21 days following adoptive NK cell transfer, BLI showed that recipients of NKG2A silenced NKL cells had slower tumor growth and significantly smaller tumor burden compared to NKL wt and NKL-GFP transduced controls (figure). Importantly, no toxicity related to infusing NKG2A inactivated NK cells was observed. These in vitro and in vivo data suggest shRNA knockdown of the NKG2A inhibitory receptor could be used as a method to augment NK cell tumor cytotoxicity in patients with hematological malignancies.
Tumor burden in mice
Luciferase-tagged 721.221 HLA-E EBV LCLs were injected into mice and imaged using a bioluminescence imager at days 10, 16, and 22 following NKL injection. 5 mice were followed in each group.
Tumor burden in mice
Luciferase-tagged 721.221 HLA-E EBV LCLs were injected into mice and imaged using a bioluminescence imager at days 10, 16, and 22 following NKL injection. 5 mice were followed in each group.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.