Abstract
Abstract 1184
Lenalidomide, a second generation immunomodulatory derivative of thalidomide, has demonstrated significant clinical benefit in myelodysplastic syndrome, multiple myeloma and B-cell malignancies. Its side effects include myelosuppression, particularly neutropenia, and impaired filgrastim induced stem cell mobilization in patients with myeloma. The precise mechanism of action of lenalidomide on hematopoietic and bone marrow microenvironment remains largely unknown. In this study, we investigated the effect of lenalidomide on survival, lineage commitment and differentiation of normal purified CD34+ hematopoietic stem cells.
Mobilized peripheral blood progenitor cells (PBPCs) were obtained from a normal allogeneic stem cell donor with an informed consent, and were subjected to positive selection with the EasySep magnetic cell selection system to purify CD34+ cells (purity of 95%). Unsorted and CD34- accessory cells were used as controls.
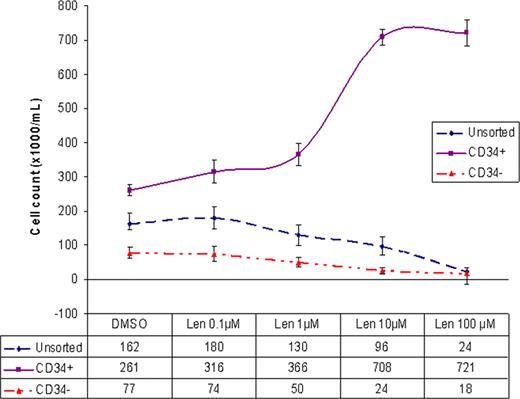
To assess the direct cytotoxic and proliferative effect of lenalidomide on purified CD34+ cells, lenalidomide (Celgene, NJ) was dissolved in DMSO and was diluted in culture medium to final concentration of 0.1, 1, 10, or 100 μM. Purified CD34+, CD34- and unsorted cells were plated in triplicates at a density of 2×104/mL in serum-free StemSpan® medium supplemented with growth factors and cytokines. 24 hours after plating the cells, lenalidomide or 0.1% DMSO as control was added. 72 hours following treatment, cells were counted with Beckman coulter counter and phenotypically analyzed using Accuri C6 flow-cytometer. The percentage of viable cells was determined using 7AAD staining. While no difference in viability was noted in the treated vs. untreated purified CD34+ cells, the viability of CD34- cells and unsorted cells was reduced to 30% and 40% respectively by lenalidomide. Contrary to our assumption, lenalidomide, in a dose dependant manner, induced proliferation of purified CD34+ cells. Maximal increase in proliferation was observed between 1mM and 10mM concentrations. The rate of growth plateaued beyond the 10mM concentration and 100mM concentration appeared to have no additional effect. At the peak of growth (10mM), the cell count nearly tripled. (Fig. 1) Lenalidomide appeared to be cytotoxic to CD34- and unsorted cells in a dose dependant manner. At 100mM concentration, the CD34- cell proliferation dropped by 4 fold and the unsorted cells by 7 fold.
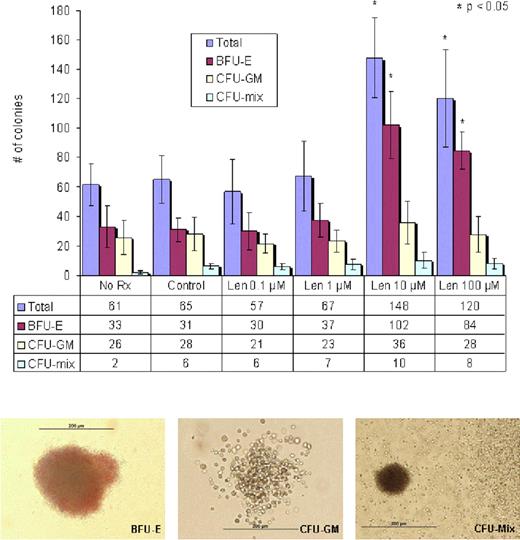
To examine the effect of lenalidomide on the clonogenic potential of purified CD34+ cells, 2,000 cells were seeded onto MethoCult® media (Stem cell technologies) supplemented with growth factors and cytokines. Cells were cultured in the presence of lenalidomide (0.1, 1, 10 and 100 μM) or 0.1 % DMSO as control. BFU-E, CFU-GM and CFU-mix were scored on day 14 with the use of an inverted microscope and standard morphological criteria. Significance of difference between groups was determined by student's t test for paired samples. Lenalidomide induced a significant increase in clonogenic activity of CD34+ cells (148 ± 27 colonies per 103 CD34+ cells, p = 0.01), most evident at the dose of 10 μM with enhanced formation of BFU-E (102 ± 23/ per 103 CD34+ cells, p = 0.01) but demonstrated no significant impact on CFU-GM (36 ± 14 per 103 CD34+ cells, p = 0.83) compared to control. (Fig. 2)
This study demonstrates that lenalidomide has pronounced effects on both CD34+ and CD34- components of mobilized PBPCs. It enhances the proliferative and clonogenic potentials of CD34+ cells with lineage commitment towards erythroid differentiation. It also has cytotoxic activity towards CD34- cells. It is likely that neutropenia and impaired mobilization of stem cells in patients treated with lenalidomide is mediated via its impact on CD34- accessory cells in the bone marrow microenvironment. Further studies exploring the impact of lenalidomide on normal bone marrow stromal cells, T and NK cell mediated signaling are needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.