Abstract
The aim of this study is to validate the clinical utility of a flow cytometric scoring system (FCSS) (1)in the diagnosis and prognosis of MDS by quantifying myeloid and monocytic dyspoiesis and correlating the accumulation of abnormalities to clinical outcome.
The BM samples were characterized by the FCSS from 56 consecutive, newly diagnosed MDS patients who were morphologically classified as RA (n=3), MDS-U (n=1), RCMD/RCMD-RS (n=32), RAEB-1 (n=12) and RAEB-2 (n=8) from January 2005 to December 2009. Three-color FCM was performed on total nonerythroid BM cells with a standardized panel of monoclonal antibodies (Table 1). The final FCSS was based on the independent analysis of data by 2 investigators (S.C.C., T.F.W.,) who were blinded to MDS/non-MDS category and clinical outcomes. The FCSS were categorized as normal/mild (0 or 1 point), moderate (2 or 3 points), or severe (4 or more points). The results of FCSS were compared with findings in 27 non-MDS cytopenic patients as the control, including diagnosis of aplastic anemia (n=7), iron deficiency anemia (n=3), folic acid or vitamin B12 deficiency anemia (n=6) and idiopathic thrombocytopenia purpura (n=11). FCSS scores were retrospectively compared with marrow blast counts, cytogenetics, hematologic parameters, IPSS and WHO categorization to assess the utility in diagnosis and prognosis of MDS. Follow-up of patients continued until May 2010, with mortality observed in 30 (54%) patients. The patient characteristics are summarized in Table 2. Nearly all MDS patients underwent supportive care rather than hypomethylation agents(n=3), chemotherapy(n=4) or haematopoietic stem cell transplantation(n=0).
The FCSS scores were significantly higher in patients with MDS than those in the non-MDS control (3 vs. 0, P < .0001). A flow score of 2 or more allowed for a specificity of 100% with 75% sensitivity in diagnosing MDS.
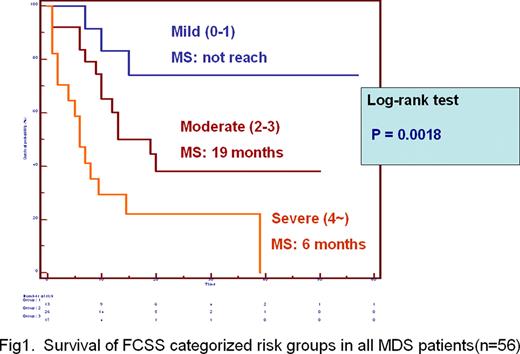
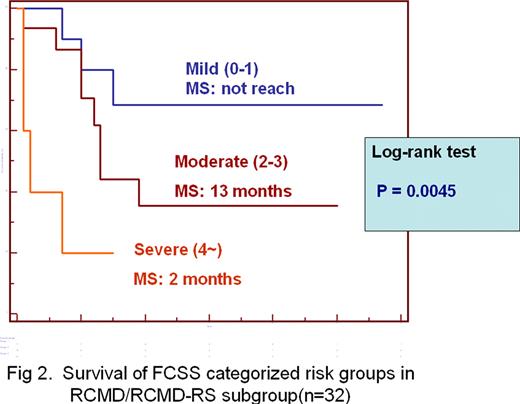
The FCSS scores correlated directly with IPSS scores (n=40, R=.5327, P =.0004) and WHO classification (n= 56, R=.5163, P<.0001) but did not correlated with IPSS cytogenetic risk categories (n=40, P= .7738). The median survival were 6 months, 19 months and not reach for MDS patients with severe, moderate and mild FCSS scores, respectively (n=56, P = .0018)(Figure 1). The Multivariate analyses using Cox proportional hazard model in a stepwise manner demonstrated the FCSS risk categories was an independent prognostic factor (P=.0020) after adjusting for sex, age (above 70 year-old or not) and IPSS risk categories. Furthermore, among 32 patients classified as RCMD/RCMD-RS, the median survival were 2 months, 13 months and not reach for patients with severe, moderate and mild FCSS scores, respectively (n=32, P = .0045) (Figure 2).
These results demonstrate that quantifying aberrancies in myelomonocytic lineage by FCSS is very useful in MDS diagnosis. As shown in a bone marrow transplant study the FCSS also predicts outcome in conventionally treated patients and may give a more accurate prognosis especially among patients classified as RCMD subgroup.
(1) Wells, DA et Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation Blood 102: 394–403, 2003.
Antibody panel
| . | FITC . | PE . | PerCP . |
|---|---|---|---|
| 1 | HLA-DR | CD11b | CD45 |
| 2 | CD5 | CD19 | CD45 |
| 3 | CD38 | CD56 | CD45 |
| 4 | CD16 | CD13 | CD45 |
| 5 | CD15 | CD34 | CD45 |
| 6 | CD14 | CD33 | CD45 |
| 7 | CD7 | CD56 | CD45 |
| 8 | HLA-DR | CD34 | CD45 |
| . | FITC . | PE . | PerCP . |
|---|---|---|---|
| 1 | HLA-DR | CD11b | CD45 |
| 2 | CD5 | CD19 | CD45 |
| 3 | CD38 | CD56 | CD45 |
| 4 | CD16 | CD13 | CD45 |
| 5 | CD15 | CD34 | CD45 |
| 6 | CD14 | CD33 | CD45 |
| 7 | CD7 | CD56 | CD45 |
| 8 | HLA-DR | CD34 | CD45 |
Patient and disease characteristics
| Characteristic . | MDS . | non-MDS . |
|---|---|---|
| No. of patients | 56 | 27 |
| Age, y [median (range)] | 73 (24–89) | 59 (19–53) |
| Sex, M/F, no. of patients | 31/25 | 16/11 |
| FCSS score [median (range)] | 3 (0–5) | 0 (0–1) |
| Follow-up, m [median (range)] | 18.5 (2–57) | |
| WHO stage, no. (%) of patients | ||
| RA/MDS-U | 4 (8%) | |
| RCMD/RCMD-RS | 32 (57%) | |
| 5q- | 0 (0%) | |
| RAEB-1 | 12 (21%) | |
| RAEB-2 | 8 (14%) | |
| IPSS risk group*, no. (%) of patients | ||
| Low | 4 (10%) | |
| Intermediate-1 | 23 (57.5%) | |
| Intermediate-2 | 12 (30%) | |
| High | 1 (2.5%) | |
| 2- or 3- lineage cytopenia, no. (%) | 40 (71%) | |
| Cytogenetic risk group*, no. (%) | ||
| Good | 18 (45%) | |
| Intermediate | 15 (37.5%) | |
| Poor | 7 (17.5%) | |
| BM myeloblast (%) by morphology, median | 4 (1–17) | |
| Ferritin level | 1171 (111–5518) | |
| AML transformation, no. (%) | 8 (14%) | |
| Median survival (months) | 14.5 (2–57) |
| Characteristic . | MDS . | non-MDS . |
|---|---|---|
| No. of patients | 56 | 27 |
| Age, y [median (range)] | 73 (24–89) | 59 (19–53) |
| Sex, M/F, no. of patients | 31/25 | 16/11 |
| FCSS score [median (range)] | 3 (0–5) | 0 (0–1) |
| Follow-up, m [median (range)] | 18.5 (2–57) | |
| WHO stage, no. (%) of patients | ||
| RA/MDS-U | 4 (8%) | |
| RCMD/RCMD-RS | 32 (57%) | |
| 5q- | 0 (0%) | |
| RAEB-1 | 12 (21%) | |
| RAEB-2 | 8 (14%) | |
| IPSS risk group*, no. (%) of patients | ||
| Low | 4 (10%) | |
| Intermediate-1 | 23 (57.5%) | |
| Intermediate-2 | 12 (30%) | |
| High | 1 (2.5%) | |
| 2- or 3- lineage cytopenia, no. (%) | 40 (71%) | |
| Cytogenetic risk group*, no. (%) | ||
| Good | 18 (45%) | |
| Intermediate | 15 (37.5%) | |
| Poor | 7 (17.5%) | |
| BM myeloblast (%) by morphology, median | 4 (1–17) | |
| Ferritin level | 1171 (111–5518) | |
| AML transformation, no. (%) | 8 (14%) | |
| Median survival (months) | 14.5 (2–57) |
Cytogenetic and IPSS risk categorizations were available in 40 patients.
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.