Abstract
Abstract 1960
Severe cardiac involvement is known to confer a poor prognosis in patients with light chain (AL-) amyloidosis. These patients do not qualify for high-dose chemotherapy regimens. In a recent analysis we could show that melphalan/dexamethasone therapy was not able to overcome this poor prognosis (Dietrich et al, 2010). Since bortezomib is known to rapidly lower the involved light chain levels (iFLC), it appears a promising alternative regimen. There is only one prospective phase I/II trial in AL amyloidosis using single agent bortezomib (Reece et al., 2009). However, patients with advanced cardiac amyloidosis had been excluded. We therefore assessed the feasibility of bortezomib/dexamethasone (Bd) in patients with high-risk cardiac amyloidosis as defined by Mayo Clinic stage III (median overall survival (OS) 3.5 months (mo), Dispenzieri et al, 2004).
We retrospectively evaluated 38 consecutive patients with systemic AL amyloidosis, who were classified as Mayo Clinic stage III cardiac amyloidosis based on their elevated cardiac biomarkers NT-proBNP, TNT and hsTNT values (patient characteristics table 1 ). 14 patients were untreated. For the sake of tolerability Bd was administered twice weekly with a reduced bortezomib dosage of 4 × 1.0 mg/m2 and dexamethasone 8 × 8 mg in a 3 week schedule.
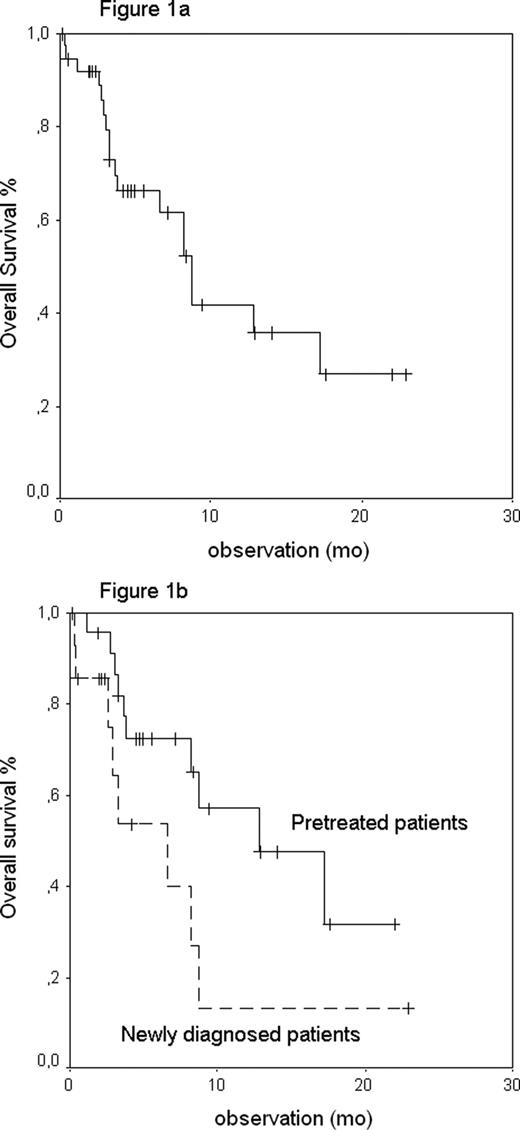
Median observation of living patients is 5 mo after start of Bd. Median OS is 9 mo (Figure 1a). 18 patients died; among them 11 (29%) during ongoing bd. Median OS of the first line therapy group was 7 mo versus 13 mo in the relapsed group (p=0.056, Figure 1b). 21 patients achieved a hematological remission (HR) after a median of 3 cycles (64% out of 33 evaluable patients, 2 CRs and 19 PRs), whereas 3 patients died during the first Bd cycle and further 2 just started with Bd. Hematologic toxicity grade 3 or 4 according to NCI criteria was observed in 9 patients (24%) (toxicities are listed in table 1 ). Apart from the 11 early deaths we observed non-hematologic toxicity grade 3 in 8 patients and grade 4 in 6 patients. In 3 patients, the bortezomib dose had to be reduced due to toxicity. Therapy was stopped before the planned 6 – 8 cycles in 11 patients, in 4 cases due to toxicity, in 5 cases due to lacking HR and in 2 cases due to patient‘s choice. 9 patients completed their therapy regularly in HR while therapy is still ongoing in 5 patients. It is planned to update these data in November 2010.
The goal of this study was to assess whether patients with severe cardiac amyloidosis as defined by Mayo Clinic stage III benefit from Bd therapy. As already known from other studies, we observed a fast reduction of iFLC within 3 months from 203 to 81 mg/l (of evaluable patients) in median. Overall, HR rate was 64%, which is comparable to 71% previously reported by Kastritis et al, 2010. However, toxicity and early death rate were considerable. Main problem of the newly diagnosed patients was the severity of cardiac involvement (median NTpro BNP 11.346 ng/l) leading to a high early mortality. Main problem of the pretreated patients was the lower HR rate of 52% versus 83% of the newly diagnosed patients. Though we observed substantial toxicities in spite of the reduced Bd dosages, the rather poor OS does not appear to be largely due to toxicity.
Dose-reduced Bd might be an important treatment option for patients with severe cardiac AL amyloidosis Mayo Clinic stage III because of its high efficacy. However, the toxicity is still significant. Inpatient care for the first cycle of Bd might lead to a reduction of the early death rate.
Patient Characteristics at start of Bd and Toxicities during treatment
| Median age (range) | 65 (47-81) years |
| Pretreatment with HDM/conventional chemotherapy | 5 pts/19 pts |
| Dialysis | 9 pts |
| NYHA stage | |
| NYHA II | 9 pts |
| NYHA III | 29 pts |
| Median NT Pro-BNP (range) in ng/l | 9.499 (663-316.187) |
| Median TNT (range) in ug/l | 0.10 (0.04-0.21) |
| Median hsTNT (range) in pg/ml | 130 (51-599) |
| Median involved FLC (range) in mg/l | 260 (88-14.300) |
| Toxicity (NCI > grade 3) | Number of Pts (%) |
| Death during therapy | 11 (29%) |
| Cardiac failure | 9 |
| Liver failure | 1 |
| Viral pneumonia | 1 |
| Non hematologic toxicity grade 3+4 | 12 (32%) |
| Polyneuropathy | 4 |
| Cardiac decompensation | 3 |
| Pneumonia | 2 |
| Viral infection | 2 |
| Renal failure | 2 |
| Muscle weakness | 1 |
| Elevated liver enzymes | 1 |
| Ileus | 1 |
| Hematologic toxicity grade 3+4 | 9 (24%) |
| Thrombocytopenia | 9 |
| Median age (range) | 65 (47-81) years |
| Pretreatment with HDM/conventional chemotherapy | 5 pts/19 pts |
| Dialysis | 9 pts |
| NYHA stage | |
| NYHA II | 9 pts |
| NYHA III | 29 pts |
| Median NT Pro-BNP (range) in ng/l | 9.499 (663-316.187) |
| Median TNT (range) in ug/l | 0.10 (0.04-0.21) |
| Median hsTNT (range) in pg/ml | 130 (51-599) |
| Median involved FLC (range) in mg/l | 260 (88-14.300) |
| Toxicity (NCI > grade 3) | Number of Pts (%) |
| Death during therapy | 11 (29%) |
| Cardiac failure | 9 |
| Liver failure | 1 |
| Viral pneumonia | 1 |
| Non hematologic toxicity grade 3+4 | 12 (32%) |
| Polyneuropathy | 4 |
| Cardiac decompensation | 3 |
| Pneumonia | 2 |
| Viral infection | 2 |
| Renal failure | 2 |
| Muscle weakness | 1 |
| Elevated liver enzymes | 1 |
| Ileus | 1 |
| Hematologic toxicity grade 3+4 | 9 (24%) |
| Thrombocytopenia | 9 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.