Abstract
Abstract 1969
High-risk MM remains a very difficult clinical challenge despite advances in therapy for the majority of patients who have benefited from the use of high-dose therapies and novel agents. Recognizing that MM from the outset represents a genomically highly complex malignancy with even further accelerated acquisition of mutations with every further relapse, we have tried to develop multi-agent combinations employing drugs with efficacy in other high-grade tumors such as large cell lymphomas and incorporated novel agents as well.
Eighty-four patients with AHRMM were given PACMED comprising cisplatin (15-25mg/m2 CI × 3d), cytarabine (1.0-1.5g/m2/d × 3), cyclophosphamide (1.0-1.5g/m2/d CI × 3), mesna (1.0-1.5g/m2/d CI × 3), etoposide (0.3-0.5g/m2/d × 3) and DEX (40-100mg/d × 3); additional agents included bortezomib (1.0-1.6mg/m2 on days 1 + 4), thalidomide (100-200mg/d × 4d) or lenalidomide (25-100mg/d × 4) and rapamycin (3mg d 1, 1mg d 2–4) with or without HPC boost. Statistical methods included Cox regression modeling for OS and EFS, along with Kaplan-Meier methodology for survival and cumulative incidence plots. Survival comparisons were made using the logrank test.
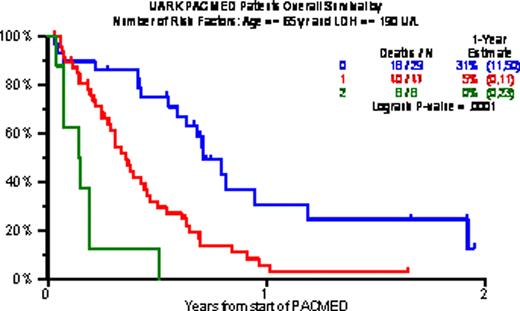
Baseline characteristics included age >=65 in 18%, B2M >=3.5mg/L in 68% and >5.5mg/L in 38%, CRP >=8mg/L in 60%, LDH >=ULN in 57%, and cytogenetic abnormalities (CA) in 62%. Gene expression profiling (GEP)-defined high-risk (70 genes, R70; 80 genes, R80) was present in 80% and 71%; PR (Proliferation), MF and MS subtypes were present in 44%, 27% and 14%. Prior transplants (Tx) had been given to 96%, including 40% who received 2Tx, 25% with 3Tx and 14% with >3Tx. PR was achieved by 29%, including 14% n-CR and 8% CR. 1-year estimates of OS and EFS were low at 13% and 8%, and median durations were 5 and 3 months. Increased age and high LDH were the only baseline characteristics adversely affecting both OS and EFS. The 29 patients with neither of these risk factors experienced 1-yr OS/EFS rates of 31%/17%; the corresponding values with 1 risk factor (n=47) were 5%/4% and with both risk factors (n=8) 0%/0%.
Single cycle PACMED provides only transient tumor control in this heavily pretreated population with 80% displaying GEP-defined high-risk and 62% CA, as a manifestation of end-stage MM. We are currently evaluating repeated cycles of PACMED earlier in the disease course in high-risk MM, in the context of a Super-BEAM transplant regimen.
Univariate and multivariate analyses for overall and event-free survival
| . | Variable . | n/N (%) . | Overall survival from start of PACMED . | Event-free survival from start of PACMED . | ||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |||
| Univariate | Age > 65 yr | 15/84 (18%) | 3.18 (1.62,6.24) | <.001 | 2.00 (1.05,3.80) | 0.035 |
| LDH > 190 U/L | 48/84 (57%) | 2.62 (1.54,4.45) | <.001 | 2.08 (1.26,3.42) | 0.004 | |
| Cytogenetic abnormalities within 1 yr of start of PACMED | 52/84 (62%) | 1.33 (0.78,2.28) | 0.293 | 1.70 (1.01,2.84) | 0.044 | |
| Multivariate | Age > 65 yr | 15/84 (18%) | 3.52 (1.79,6.92) | <.001 | 2.20 (1.15,4.22) | 0.017 |
| LDH > 190 U/L | 48/84 (57%) | 2.75 (1.62,4.67) | <.001 | 2.17 (1.32,3.58) | 0.002 | |
| . | Variable . | n/N (%) . | Overall survival from start of PACMED . | Event-free survival from start of PACMED . | ||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |||
| Univariate | Age > 65 yr | 15/84 (18%) | 3.18 (1.62,6.24) | <.001 | 2.00 (1.05,3.80) | 0.035 |
| LDH > 190 U/L | 48/84 (57%) | 2.62 (1.54,4.45) | <.001 | 2.08 (1.26,3.42) | 0.004 | |
| Cytogenetic abnormalities within 1 yr of start of PACMED | 52/84 (62%) | 1.33 (0.78,2.28) | 0.293 | 1.70 (1.01,2.84) | 0.044 | |
| Multivariate | Age > 65 yr | 15/84 (18%) | 3.52 (1.79,6.92) | <.001 | 2.20 (1.15,4.22) | 0.017 |
| LDH > 190 U/L | 48/84 (57%) | 2.75 (1.62,4.67) | <.001 | 2.17 (1.32,3.58) | 0.002 | |
Overall and event-free survival
Cumulative incidence of response
Overall survival by number of risk factors (Age > 65 yr, LDH > 190 U/L)
Overall survival by number of risk factors (Age > 65 yr, LDH > 190 U/L)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.