Abstract
Abstract 1979
Polycythemia vera (PV) is an acquired clonal blood disorder characterized by variable increased number of clonal myeloid cells (erythrocytes, granulocytes and platelets) and mostly normal polyclonal T lymphocytes. Most patients carry a gain-of function JAK2 V617F mutation in 2 copies acquired by a uniparenteral disomy; i.e. a copy-neutral loss of heterozygosity (LOH) in chromosome 9p in PV pathogenesis. Yet, JAK2 V617F mutation is not a PV-initiating event and the family clustering of PV suggests a contribution of inherited genetic events to its pathogenesis. Interestingly, majority of PV subjects acquire the JAK2 V617F mutation on a distinct a germline SNP 9p24.1 haplotype. Using whole-genome SNP arrays, we assayed 34 T-cells and 66 granulocytes from PV patients (including 32 pairs from the same patient). Comparison to a control population identified multiple germline SNPs around JAK2 as being associated with PV susceptibility (rs11999802, P=1.8×10-8, OR=4.4). Analysis of somatic genomic changes in granulocytes revealed strong genetic heterogeneity: we detected 9p LOH in 36% of the patients, 9p chromosomal gain in 1 patient, as well as “fractional LOH” in 14% of the patients where a small fraction of granulocytes have LOH. Interestingly, the magnitude of somatic LOH in 9p was strongly associated with somatic V617F allelic dosage (r=0.74, P=4.8×10-12), suggesting that LOH preferentially increase V617 PV subclone without altering genomic copy number. Adjusting for the fractional LOH, we did not have sufficient power to detect association between rs11999802 germline genotypes and increased dosage of somatic V617F mutations. Analysis of granulocytes with heterozygous rs11999802 genotypes demonstrated that LOH increases the relative fraction of germline risk alleles versus non-risk alleles. In summary, germline risk variants surrounding JAK2 predispose to somatic SNVs within JAK2, whose allelic dosage can be further increased by a serial subclonal expansion of allele-specific copy-neutral LOH or chromosomal duplications. Our study illustrates how convergent mechanisms of somatic mutations increase the genetic load of gain-of-function mutations contributing to the clonal expansion and disease pathogenesis.
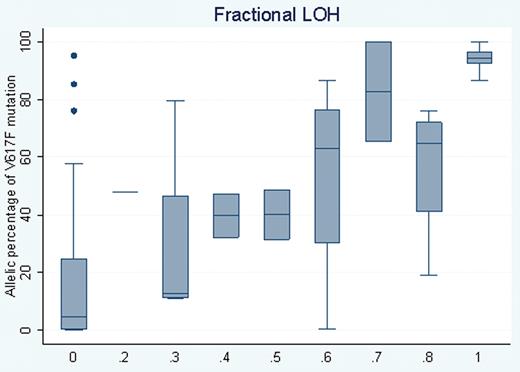
Box plot of the percentage of somatic V617F mutation in JAK2 over each of the fractional LOH measures in granulocytes. Strong correlation between these two measures was detected (r=0.74, P=4.8×10-12).
Box plot of the percentage of somatic V617F mutation in JAK2 over each of the fractional LOH measures in granulocytes. Strong correlation between these two measures was detected (r=0.74, P=4.8×10-12).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.