Abstract
Abstract 2338
Whatever virus specie, Influenza infection is a significant cause of morbidity and mortality, particularly in immuno-compromized patients. In 2009, a new H1N1 virus appeared in Mexico and spread rapidly all around the world. This new virus is unrelated to the human seasonal H1N1 viruses that have been in general circulation among people since 1977. At fall 2009, hematopoietic-stem cell transplant (HSCT) recipients were considered at high risk of severe Influenza infection with this new virus. Then, both French and European Bone Marrow Transplantation societies counselled both seasonal and pandemic-H1N1 vaccinations after HSCT sooner than usual vaccination schedule despite the absence of data regarding safety and immunogenicity. Immunity against Influenza is based on specific antibodies but need also specific functional T-cells. In this study we investigated T-cell immunogenicity to pandemic H1N1 vaccination in pediatric allogenenic HSCT recipients.
39 HSCT recipients transplanted from Jan'08 to Dec'09 were included (see table 1). There were 26 males and 13 females. Median age was 6.1y (range: 0.6–16.6). Twenty-two patients (pts) received 1 to 2 doses of either adjuvanted or non-adjuvanted EMEA certified pandemic-H1N1 vaccine at median of 9.5 months post-HSCT (range:2-23) and 17 pts did not. Underlined diseases were acute leukemia for 29 pts, JMML for 5, SAA for 2 and other for 3. All pts but 2 received myelo-ablative conditioning regimen. Vaccinated pts were older than non-vaccinated (median age 9.1y vs 3.1, respectively). Seven vaccinated immuno-competent adults were also investigated as controls. T-cell immunogenicity was evaluated for proliferative responses to in vitro stimulation with an inactive form of H1N1 virus. T- cell proliferative-responses to cytomegalovirus (CMV) and adenovirus (CMV) were also evaluated for both vaccinated or not-vaccinated pts.
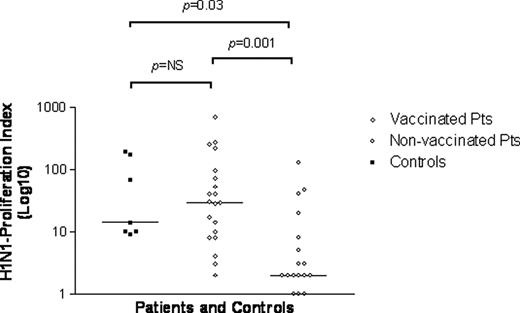
Results show significantly higher proliferative-responses to H1N1 in vaccinated than in non- vaccinated HSCT recipients (median cpm: 30310 versus 2479, respectively; p<0.005 and median stimulation index: 29.5 versus 2, respectively; p<0.001) (see figure 1). Moreover, intensity levels of proliferative-responses were similar in vaccinated HSCT recipients and vaccinated adult controls. The two patient-groups exhibit same specific T-cell proliferation level for both CMV and ADV. Then differences for specific H1N1 T-cell proliferative response between vaccinated pts and non-vaccinated could be linked to vaccinal status and not to global immune status. None patient but one presented any adverse event after vaccine injection other than slight fever or local pain. An 11 year-old girl presented with extensive cutaneous cGvHD developed high fever and severe skin inflammation extended to left arm and was hospitalized for IV antibiotics during 4 days after vaccine injection without any sequelae nor GvHD extension.
In conclusion, H1N1 vaccine can be performed safely soon after HSCT whatever the type of used conditioning regimen and transplant. This vaccination induces clear CD4 T-cell responses even after a single dose administered as soon as D60 post-HSCT in paediatric recipients. It was demonstrated that efficient immunity against Influenza virus is mediated by antibodies. Antigenic analysis has shown that antibodies to the seasonal H1N1 virus do not protect against the pandemic H1N1 virus. Then specific new-H1N1 antibody dosages are on-going in our pts.
| . | Vaccinated HSCT recipients . | Non vaccinated HSCT recipients . | Total . |
|---|---|---|---|
| 22 | 17 | 39 | |
| Median age at HSCT (y) | 8,25 [0,41–15,92] | 2,58 [0,41–15,33] | 5,75 [0,41–15,92] |
| Time from HSCT to 01.01.10 (months) | 10 [2–23] | 5,63 [0,33–23] | 7,63 [0,33–23] |
| Gender (M/F) | 14/8 | 12/5 | 26/13 |
| Stem cell sources | |||
| BM | 162 | 11 | 27 |
| PBSC | 2 | 2 | 4 |
| CB | 4 | 4 | 8 |
| Donor type | |||
| Sibling | 12 | 5 | 17 |
| Haplo | 2 | 0 | 2 |
| Matched unrelated donor (9-10/10) | 4 | 8 | 12 |
| Unrelated CB (4-6/6) | 4 | 4 | 8 |
| aGVHD ≥grade 2 at vaccination (n) | 1 | ||
| cGVHD at vaccination (n) | 6 | ||
| Therapy for aGVHD or cGVHD at vaccination (n) | 6 |
| . | Vaccinated HSCT recipients . | Non vaccinated HSCT recipients . | Total . |
|---|---|---|---|
| 22 | 17 | 39 | |
| Median age at HSCT (y) | 8,25 [0,41–15,92] | 2,58 [0,41–15,33] | 5,75 [0,41–15,92] |
| Time from HSCT to 01.01.10 (months) | 10 [2–23] | 5,63 [0,33–23] | 7,63 [0,33–23] |
| Gender (M/F) | 14/8 | 12/5 | 26/13 |
| Stem cell sources | |||
| BM | 162 | 11 | 27 |
| PBSC | 2 | 2 | 4 |
| CB | 4 | 4 | 8 |
| Donor type | |||
| Sibling | 12 | 5 | 17 |
| Haplo | 2 | 0 | 2 |
| Matched unrelated donor (9-10/10) | 4 | 8 | 12 |
| Unrelated CB (4-6/6) | 4 | 4 | 8 |
| aGVHD ≥grade 2 at vaccination (n) | 1 | ||
| cGVHD at vaccination (n) | 6 | ||
| Therapy for aGVHD or cGVHD at vaccination (n) | 6 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.