Abstract
Abstract 2388
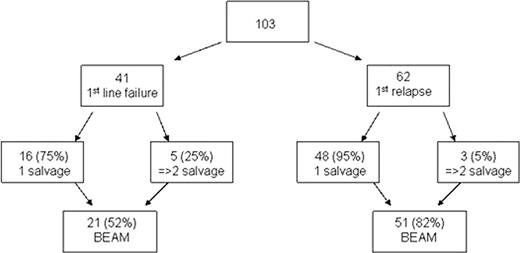
High dose chemotherapy with autologous stem cell rescue (HDT-ASCR) currently offers patients with classical Hodgkin lymphoma (CHL) with primary refractory or relapsed disease the best chance of cure, particularly when offered in complete response (CR) following salvage therapy. Treatment of chemotherapy-refractory patients remains contentious and is associated with poor outcomes and high relapse rates. At St Bartholomew's Hospital, London (SBH), HDT-ASCR with BEAM (BCNU, etoposide, Ara-C and melphalan) has been administered only to patients who have demonstrated CR or partial response (PR) to salvage chemotherapy. We performed a retrospective intention to treat analysis on all patients for whom consolidation with BEAM as HDT-ASCR was planned between 1995 and 2008 to assess the success of this approach. Eligible patients with CHL were identified as those achieving less than CR to first-line chemotherapy (first-line failure), or those who had relapsed fewer than 5 years after first CR, were 18–60 years of age at the time of salvage therapy, and HIV negative. Initial chemotherapy comprised an anthracycline-containing regimen in all cases. 103 patients were identified (62% male, median age 31, range 18–59, median follow up 8 years, range 2–15 years). HDT-ASCR indications were first-line failure in 41 (39%) and first relapse in 62 (61%). Of the 41 patients in the first-line failure group, only 52% proceeded successfully to BEAM having demonstrated chemosensitivity: 75% following single-line salvage, [predominantly with ChlVPP (chlorambucil, vincristine, procarbazine and prednisolone) in 60% or gemcitabine-based chemotherapy in 20%], whereas 25% required two different lines of salvage chemotherapy. Of the 62 patients treated at first relapse, 82% proceeded successfully to BEAM, 95% following single-line salvage, predominantly with ChlVPP in 55%, VAPEC-B (vincristine, doxorubicin, prednisolone, etoposide, cyclophosphamide and bleomycin) in 20% and gemcitabine-based regimens in 10%. Only 5% required 2 or more lines of salvage (Figure 1). Overall survival (OS) at 5 years for all patients by intention to treat with HDT-ASCR was 63% (Figure 2); 76% for the relapse group, and 45% for the first line failure group. In the cohort who successfully received BEAM chemotherapy, OS was 75% at 5 years (with no significant survival difference between the relapsed and the first line failure groups) with a 100-day treatment-related mortality of 4%, the other deaths being from relapsed CHL except for one case of secondary malignancy occurring 15 years after BEAM. Salvage of CHL in relapse, or in those who have failed to achieve CR with first-line therapy remains an area in which improvements need to be made. There are few published analyses of outcome by intention to treat with HDT-ASCR. The relatively conservative strategy at SBH of offering HDT-ASCR only to those with chemosensitive disease at salvage has lead to excellent outcomes in the more selected population reaching HDT-ASCR, without compromising overall outcomes of the eligible population.
Gribben: Roche: Consultancy; Celgene: Consultancy; GSK: Honoraria; Napp: Honoraria. Lister: Allos: Consultancy; Bioconnections: Consultancy; Astra Zeneca: Consultancy; Tenet: Consultancy; GSK: Chairman of Safety Monitoring Committee.
Author notes
Asterisk with author names denotes non-ASH members.