Abstract
Abstract 2409
Serum free light chain (FLC) quantitation has prognostic value in MGUS, multiple myeloma (MM), solitary plasmacytoma, and amyloid light chain (AL) amyloidosis, and it has been incorporated in response criteria for both MM and AL amyloidosis. Two recent studies have shown FLC abnormalities are present in 39–54% of CLL patients and are associated with shorter time to treatment (TTT) and inferior overall survival (OS) in CLL. Here we report the largest study to date of FLC abnormalities in a prospective cohort of CLL patients and explore the effects of both monoclonal and polyclonal FLC elevations.
Newly diagnosed CLL patients were prospectively enrolled in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER). Serum FLC was quantitated from stored research serum samples using the Freelite FLC assay (The Binding Site, Ltd., Birmingham, UK). Elevated FLC was defined as either kappa or lambda above the reference range (k>19.4 mg/L, l>26.3 mg/L). Monoclonal FLC elevation was defined as elevated FLC with an abnormal FLC ratio. Polyclonal FLC elevation was defined as elevated FLC with a normal FLC ratio (0.26–1.65). Patients with normal FLC but abnormal FLC ratio were evaluated as a distinct group (Abnormal FLC Ratio). Clinical data was abstracted using a standard protocol for the MER and patients were followed systematically for TTT and OS. Cox proportional hazards models were used to assess the association of FLC with TTT and OS.
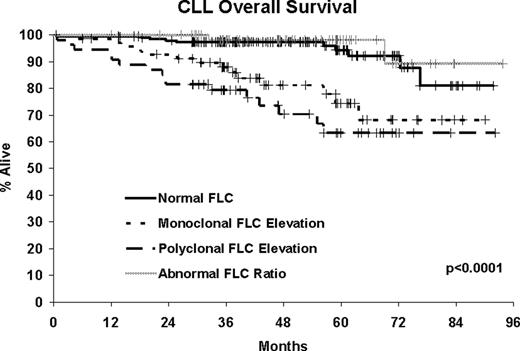
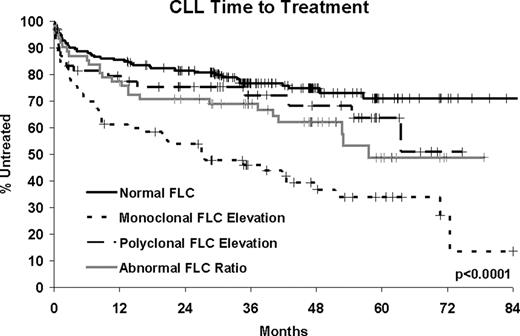
385 CLL patients diagnosed between September 2002 and February 2008 with available serum were assayed. At a median follow-up of 47 months (range 1–94), 135 patients (35%) have been treated and 43 patients have died (11%). Abnormalities in FLC were present in nearly half of the CLL patients (48.8%). Although 32.4% had an elevated kappa or lambda, only 14.0% had a monoclonal FLC elevation while the remaining 18.4% had a polyclonal FLC elevation. An additional 16.4% had an abnormal ratio without elevated FLC. Monoclonal FLC elevation was associated with high risk features of CD38+, CD49D+, ZAP70+, IGHV unmutated and having high risk FISH (del 17p13; del 11q22). Monoclonal FLC elevation was highly associated with shorter TTT (HR=3.24, p=0.0007) and poor overall survival (HR=4.14, p=0.0006). These associations remained statistically significant after adjusting for stage and prognostic factors. Abnormal FLC ratio without elevated FLC was also associated with shorter TTT (HR=1.74, p=0.03), though this association did not hold after adjustment for stage and prognostic factors. Polyclonal FLC elevations were associated high risk FISH and poor overall survival (HR=6.41, p<0.0001, adjusted HR = 4.82, p=0.0002) but not with TTT.
In the largest series of newly diagnosed CLL patients reported to date, nearly half of CLL patients have abnormal FLC concentrations. Monoclonal FLC elevation is an independent predictor of shorter TTT and inferior overall survival in CLL, is associated with high risk markers, and may be a marker of an aggressive tumor. Polyclonal FLC elevation without abnormal FLC ratio is independently associated with poor overall survival but not TTT suggesting it may be a non-specific marker of host frailty/comorbidity rather than disease specific outcomes. Regardless of cause, CLL patients with elevated FLC have poor outcome and FLC may have utility as a biomarker in CLL.
| . | N . | CD38+ . | CD49D+ . | ZAP70+ . | IGHV Unmutated . | High Risk FISH . | Rai Stage 0/1/2–4 . |
|---|---|---|---|---|---|---|---|
| Normal FLC | 197 | 19.1% | 23.7% | 23.9% | 28.7% | 7.0% | 53.3%/42.1%/4.6% |
| Monoclonal FLC Elevation | 54 | 48.4% | 50.0% | 55.0% | 58.3% | 19.6% | 29.6%/47.9%/22.5% |
| Polyclonal FLC Elevation | 71 | 31.8% | 29.2% | 19.5% | 38.2% | 25.6% | 47.2%/43.4%/9.4% |
| Abnormal FLC Ratio (no elevation) | 63 | 26.8% | 35.3% | 32.0% | 38.9% | 12.2% | 26.0%/39.7%/14.3% |
| . | N . | CD38+ . | CD49D+ . | ZAP70+ . | IGHV Unmutated . | High Risk FISH . | Rai Stage 0/1/2–4 . |
|---|---|---|---|---|---|---|---|
| Normal FLC | 197 | 19.1% | 23.7% | 23.9% | 28.7% | 7.0% | 53.3%/42.1%/4.6% |
| Monoclonal FLC Elevation | 54 | 48.4% | 50.0% | 55.0% | 58.3% | 19.6% | 29.6%/47.9%/22.5% |
| Polyclonal FLC Elevation | 71 | 31.8% | 29.2% | 19.5% | 38.2% | 25.6% | 47.2%/43.4%/9.4% |
| Abnormal FLC Ratio (no elevation) | 63 | 26.8% | 35.3% | 32.0% | 38.9% | 12.2% | 26.0%/39.7%/14.3% |
| Cox Models . | Unadjusted . | Adjusted for Stage and Prognostic Factors . | ||||||
|---|---|---|---|---|---|---|---|---|
| TTT HR . | TTT 95% CI . | OS HR . | OS 95% CI . | TTT HR . | TTT 95% CI . | OS HR . | OS 95% CI . | |
| Normal FLC | 1.00 | REF | 1.00 | REF | ||||
| Monoclonal FLC Elevation | 3.24 | 2.15, 4.89 | 4.14 | 1.84–9.33 | 1.82 | 1.19–2.78 | 2.67 | 1.15–6.19 |
| Polyclonal FLC Elevation | 1.45 | 0.83, 2.52 | 6.41 | 2.91–14.1 | 1.19 | 0.68–2.07 | 4.82 | 2.11–11.0 |
| Abnormal FLC Ratio (no elevation) | 1.74 | 1.07, 2.83 | 0.57 | 0.13–2.62 | 1.24 | 0.76–2.04 | 0.60 | 0.13–2.73 |
| Cox Models . | Unadjusted . | Adjusted for Stage and Prognostic Factors . | ||||||
|---|---|---|---|---|---|---|---|---|
| TTT HR . | TTT 95% CI . | OS HR . | OS 95% CI . | TTT HR . | TTT 95% CI . | OS HR . | OS 95% CI . | |
| Normal FLC | 1.00 | REF | 1.00 | REF | ||||
| Monoclonal FLC Elevation | 3.24 | 2.15, 4.89 | 4.14 | 1.84–9.33 | 1.82 | 1.19–2.78 | 2.67 | 1.15–6.19 |
| Polyclonal FLC Elevation | 1.45 | 0.83, 2.52 | 6.41 | 2.91–14.1 | 1.19 | 0.68–2.07 | 4.82 | 2.11–11.0 |
| Abnormal FLC Ratio (no elevation) | 1.74 | 1.07, 2.83 | 0.57 | 0.13–2.62 | 1.24 | 0.76–2.04 | 0.60 | 0.13–2.73 |
Zent: Genzyme: Research Funding; Genentech: Research Funding; Novartis: Research Funding; G.S.K.: Research Funding.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.