Abstract
Abstract 2765
CD4+CD25+FOXP3+ regulatory T cells (Tregs) employ a range of suppressive strategies including factors that are cytotoxic to target CD4+CD25−FOXP3− effector T cells (Teff) such as perforin and granzyme B and those that suppress proliferation, differentiation and/or cytokine production including TGF-beta1, IL-10 and CTLA-4. The relative contribution of each of these mechanisms to Treg function is unclear but from the available data their importance appears context specific. Because T cells are very sensitive to the redox state of the microenvironment, and display a pattern of impaired activation under conditions of oxidative stress, we investigated the potential contribution of an oxidant dependent suppressive pathway on direct Treg mediated suppression of Teff in vitro using the antioxidant n-acetylcysteine (NAC) to block reactive oxidants.
Tregs and Teff were derived from the spleens of 2–4 month old C57BL/6 mice maintained in pathogen free conditions. T cell subsets were isolated using magnetic bead based techniques. Purity was assessed by flow cytometry. For suppression assays, Tregs were co-cultured with CFSE-labeled Teff at the indicated ratios and stimulated with anti-CD3/CD28 coated beads for 3 days. Proliferation was measured by flow cytometric evaluation of CFSE dilutional staining. Suppression was calculated by comparing proliferation of Teff cultured alone to those co-cultured with Tregs (% suppression= 1 − Tregs:Teff/Teff alone). In other experiments, CFSE-labeled Teff were cultured alone in the indicated conditions with TGF-beta +/− NAC and proliferation was assessed by CFSE staining. Intracellular reactive oxygen species (ROS) were measured by flow cytometry using the redox sensitive cell permeable indicator dye 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (DCFDA).
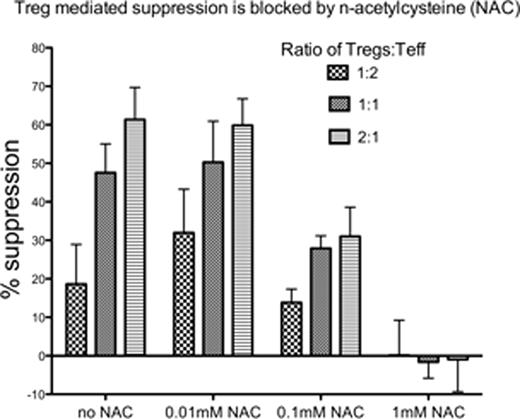
The presence of NAC prevented Treg suppression of Teff in a dose dependent fashion at Treg:Teff ratios of 1:2, 1:1 and 2:1 (see figure). Suppression was significantly decreased at 0.1mM NAC (p<0.05 at 1:1; p<0.01 at 2:1) and essentially absent at 1mM NAC. Proliferation of Teff was markedly higher in the setting of 1mM NAC compared to conditions without NAC, even during co-culture with Tregs at a 2:1 ratio of Tregs:Teff. Treg suppression of Teff proliferation was dependent on TGF-beta as neutralizing antibodies reversed the effect (p<0.001). The presence of NAC was sufficient to overcome the suppressive effects of exogenous TGF-beta on CD3/CD28 stimulated Teff proliferation. Treatment of CD3/CD28 stimulated Teff with TGFbeta resulted in a significant dose dependent increase in the levels of intracellular ROS (p<0.0001 at 10ng/mL TGF-beta) that inversely correlated with the degree of proliferation.
NAC blocks Treg mediated TGF-beta dependent suppression in vitro. This suggests that TGF-beta may function to suppress proliferation of Teff via a ROS dependent mechanism and raises the possibility that targeted delivery of antioxidants may have clinical utility for modulating the effects of Tregs in vivo.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.