Abstract
Abstract 2777
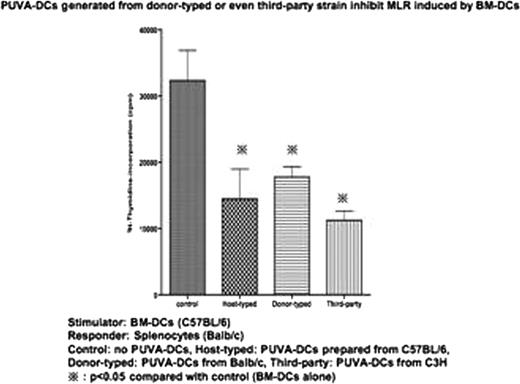
We have reported that bone marrow derived dendritic cells with psoralen and UVA (PUVA-DCs) treatment acquired tolerogenicity in mice. With the purpose of potential application of PUVA-DCs in a clinical hematopoietic stem cell transplantations (HSCT) for graft-versus-host disease (GVHD), we showed that mixed lymphocyte reaction (MLR) was strongly inhibited when PUVA-DCs from the stimulator strain were added to the coculture (Stimulator (S): conventional DCs obtained from C57BL/6, Responder (R): splenocytes obtained from Balb/c, PUVA-DCs: C57BL/6). This suggests that infusion of host-typed PUVA-DCs would become a novel therapeutic approach for GVHD. However utilizing host-typed DCs has problems because of leukemic cell contaminations or low efficiency of cell culture from the patients receiving repetitive chemotherapy. Therefore next concern is whether PUVA-DCs generated from BM donor or even strangers would have same tolerogenicity as host-typed PUVA-DCs do. To test this, we performed MLR by adding PUVA-DC generated from the same strain of responder or third party strain (S: conventional DCs obtained from C57BL/6, R: splenocytes obtained from Balb/c, PUVA-DC: C57BL/6 or C3H). Proliferation was significantly inhibited when PUVA-DC generated from the stimulator strain were added to the coculture (p<0.05). Also significant inhibition was observed (p<0.05) when adding PUVA-DCs generated from third party, suggesting that PUVA-DCs have tolerogenicity in a MHC-independent manner.
To clarify the mechanisms of how PUVA-DCs induce tolerogenicity, we performed MLR as mentioned above with the addition of neutralizing antibodies against IL-10 or TGF-beta1 or both, which have immunosuppressive effects. Neutralization of immunosuppressive cytokines had no effects on MLR. We then hypothesized that cell-to-cell contact between PUVA-DCs and alloreactive T-cells was needed to mediate the regulatory effect. To this end, we performed MLR using transwell to prevent cell-to cell contact. MLR was not suppressed when transwell was used, suggesting that PUVA-DCs dominantly regulates the alloreaction in a cell contact-dependent manner.
This is the first report that PUVA-DCs prepared not only from host-typed but from donor-typed or even third-party could induce strong inhibition of alloreaction. Tolerogenic DCs prepared previously by several ways could not induce inhibition of alloreaction in vitro when these cells were prepared from donor-typed or third-party strains in mice. To apply tolerogenic dendritic cells for GVHD in clinical settings, it is necessary to obtain sufficient doses of PUVA-DCs with ease and safety guaranteed. Therefore in the future PUVA-DCs generated even from HLA mismatched iPS cells would be a promising approach. In conclusion, infusion of PUVA-DCs from donor-typed or even third party strain could have a potent strategy for treatment of lethal GVHD and autoimmune diseases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.