Abstract
Abstract 2972
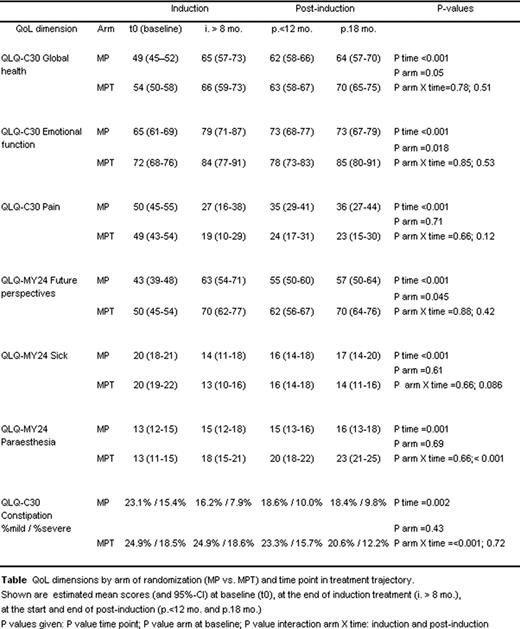
Thalidomide (T) with melphalan/prednisone (MPT) was defined as a standard treatment in elderly patients with multiple myeloma (MM) based on 5 randomized trials. Treatment with MPT not only showed improved response rate, significantly better time to response as well as quality of response but also a significant improvement of event free survival (EFS), progression free survival (PFS) and overall survival (OS). In one of these trials, HOVON 49, a prospective Health related – Quality of life (HRQoL) was initiated in order to asses the impact of T on QoL. (Wijermans et al, J Clin Oncol 28:3160-6). Patients aged >65 years with newly diagnosed symptomatic MM were randomized to receive 8 cycles of MP or MPT, followed by T maintenance in MPT arm. 284 patients were included in this HRQoL side study (MP: n=149, MPT: n=135). HRQoL was assessed with the EORTC core QoL Questionnaire (QLQ-C30) and the myeloma-specific module (QLQ-MY24) at baseline and at pre-determined intervals during treatment. Treatment-related toxicity WHO grade 2–4 occurred in 60% of MP and 87% of MP-T treated patients. Most frequently found were neutropenia-related infections, neurotoxicity (mostly T induced peripheral neuropathy) and myelotoxicity.
The QLQ-C30 subscales Physical Function (p=0.044) and Constipation (p<.001) showed a treatment related improvement during induction in favour of the MP arm. During T maintenance, the scores for the QLQ-MY24- Paraesthesia became significantly higher in the MPT arm (p<.001). The QLQ-C30 subscales Pain (p=0.12), Insomnia (p=0.068), Appetite loss (p=0.074) and the QLQ-MY24- item Sick (p=0.086) scored marginally better during T maintenance. The overall QoL scale QLQ-C30-HR-QOL showed a significant time trend towards more favourable mean values during protocol treatment but did not reveal differences between MP and MPT. For the QLQ-C30 subscales Emotional function and Future perspectives, difference in favour of the MPT arm from the start of treatment was observed (p=0.018and p=0.045 respectively) with no significant ‘time × arm' interaction, indicating a persistent better patient perspective with MPT treatment. Since the first questionnaire was filled out prior to treatment but after randomization we hypothesise that the awareness of being treated with a medication that holds out a prospect of better treatment outcome may in itself be associated with improved feeling of well-being and hope for the future.
This prospective study shows that the higher frequency of adverse effects associated with MPT does not translate into a negative effect on HRQoL and that MPT holds a better patient perspective.
No relevant conflicts of interest to declare.

This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.