Abstract
Abstract 3299
Pre-clinical studies suggest that mammalian target of rapamycin (mTOR) is important for leukemic stem cell proliferation and self-renewal. Rapamycin produces short-lived leukocyte reductions in advanced AML (Boehm et al, Euro J. of Int. Med 2009). Everolimus (RAD001) is an orally available ester derivative of the macrolide antifungal antibiotic sirolimus (rapamycin). As a single agent, everolimus is tolerated at a daily dose of 10 mg daily and demonstrates modest clinical activity in myelodysplastic syndrome (Yee et al, Clin Cancer Res 2006). Rapamycin synergistically kills AML cells when combined with cytarabine in vitro (Janus et al, Anti-Cancer Drugs 2009).
To determine the safety and preliminary efficacy of everolimus in combination with low dose cytarabine (LDAC) in untreated, elderly AML unfit for intensive chemotherapy.
Phase Ib open label dose escalation study. Patients were treated with LDAC 20 mg s.c. twice daily on days 1–10 of each 28-day cycle for a maximum of 4 cycles and either 2.5, 5 or 10 mg everolimus per orally daily on days 1–28 for a maximum of 12 cycles. 8 patients were treated at each everolimus dose level.
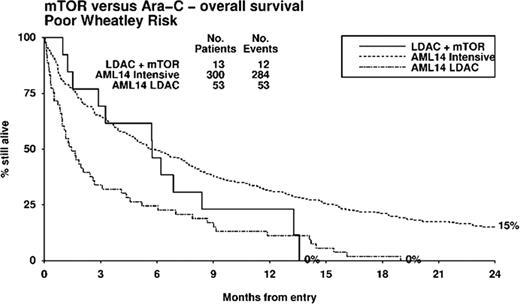
24 patients (M 14, F 10), median age 74 years (range 62–86), were included in the study. 71% had at least one poor risk factor defined by age >75 (33%), ECOG>2 (13%), secondary AML (38%) or adverse risk cytogenetics (25%). During cycle 1, grade 4 thrombocytopenia occurred in 58% and grade 4 neutropenia in 67%. Grade 3/4 non-hematologic toxicity occurred in 6 (25%) patients; 2.5 mg everolimus (n=1; flare leucocytosis), 5 mg everolimus (n=1; peripheral edema) and 10 mg everolimus (n=4; cough, mouth ulcers and septicemia-2). The maximum tolerated dose of everolimus was 5 mg in combination with LDAC. The most frequently delivered dose of everolimus was 2.5 mg (46 cycles), compared to 5 mg (31 cycles) and 10 mg (17 cycles). After 2 cycles, the overall response rate (ORR) was 34% (CR 13%, CRi 4% and PR 17%). Stable disease was observed in 50%, while 4% had progressive disease. ORR was 50%, 13% and 38%, respectively in the 2.5, 5 and 10 mg everolimus cohorts. 30 and 60-day mortality was 8 and 25%, respectively. Median overall survival for the entire cohort was 179 days. One and two-year survival was 29% and 18%, respectively. Median overall survival was superior in responders (not reached) compared to non-responders (142.5 days; p=0.005). There was no significant difference in overall survival between the 3 everolimus dosing cohorts (p=0.9). The median progression free survival was 76 days (range 13–475). Two patients in CR relapsed within 2 to 4 months after ceasing everolimus at 12 months. A stratified analysis was performed comparing elderly unfit AML treated with LDAC on the UK Leukemia Research Fund AML14 study (Burnett et al, Cancer 2007) adjusting for possible imbalances in risk factors. The Wheatley index is a validated score used to define good, standard and poor risk elderly AML groups (Wheatley et al, Brit. J. Haematol. 2009). Patient groups were similar in demographics, with no significant differences by Wheatley risk group (LDAC+everolimus vs LDAC: good 21 vs 10%; standard 25 vs 28%; poor 54 vs 61%; p=0.3). In analyses stratified for Wheatley risk, LDAC + everolimus gave non-significantly better outcomes (HR 0.74, 95% CI 0.46 to 1.19, p=0.2). In poor risk patients, median survival was superior with LDAC + everolimus vs LDAC alone (175 days vs 44 days), and interestingly, survival was not significantly different to poor risk patients treated with intensive chemotherapy (median survival 175 days, p=0.3, Figures 1).
In an unfit elderly AML population, everolimus in combination with LDAC followed by everolimus maintenance therapy was tolerable and active, with extended survival observed in those achieving hematologic response. Further evaluation is warranted in randomised trials.
Wei:Novartis: Advisory board, Research Funding. Off Label Use: AML. Catalano:Celgene: Research Funding; Roche: Honoraria, Research Funding, Travel Grants.
Author notes
Asterisk with author names denotes non-ASH members.