Abstract
Abstract 3810
Despite frequent anemia and multiple transfusions during AML chemotherapy and allogeneic hematopoietic stem cell transplantation (allo-HSCT), recommendations and marketing authorization for erythropoietin (EPO) use are still missing. In the current prospective study, as primary objective, we evaluated the effect of EPO on patient's quality of life (QOL). Secondary objective was hemoglobin (Hb) recovery. In addition, a paired matched analysis using similar population was conducted to compare platelets (Pt) and red blood cells (RBC) transfusion number.
We included adult patients with Hb level ≤11g/dl induced by 1, 2 or 3 consolidation chemotherapy for AML in complete remission (CR) (group 1); or by allo-HSCT for any hematological disease (group 2). EPO was administered Sc. once per week during a maximum period of 6 months: for group 1, ARANESP® 150μg; for group 2, NEORECORMON® 30000IU; Hb level was monitored every week. Injections were stopped once the Hb level reached 12g/dl without any transfusion. If after 4 injections, no improvement was observed, doses were doubled, and if after 8 injections, no improvement was observed, patient was taken off-study for EPO inefficiency. The QOL was measured at baseline, at 1, 2, 3 and 6 months by the Functional Assessment of Cancer Therapy–Anemia (FACT–An). EPO responders patients were defined as having Hb level ≥12g/dl (EPO CR) or a ≥ 2g/dl increase [EPO partial response (EPO PR)] compared with baseline value without any transfusion requirement. The matching analysis took into account: sex, age, disease status, for the two groups, associated to cytogenetics, type of chemotherapy, sequential chemotherapy number for group 1, and diagnosis, conditioning, HSC source, number of previous transplants and GVHD for group 2.
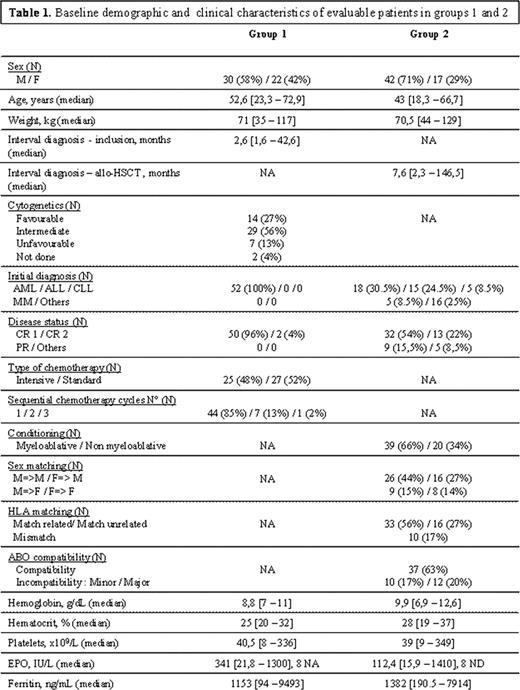
Between April 2006 and December 2009, among 261 screened patients, 55 were included in group1 and 61 in group 2, patient characteristics for each group are summarized in Table1. Main exclusion criteria were EPO contra-indication and patient refusal. The median number of EPO injections/patient was 13 (3 – 24) in group1 and 8 (2 - 28) in group 2. For the global population (111 evaluable patients [52 group1 and 59 in group 2]), we have noticed a significant improvement of QOL during the 6 months follow-up according to FACT-An anemia (p=0.01). Despite a non-significant improvement for FACT-G, we observed a significant improvement in physical well-being (p<0.0001). There were 85 EPO CR (83% in group1 and 71% in group 2) and 3 (6%) EPO PR (only in group1). Among patients who reached the 6 months follow-up, 81% had a normal Hb level. Fourteen patients (13%) were withdrawn (6 in group1 and 8 in group 2) due to EPO inefficacy and 9 in group 2 for relapse or EPO related/unrelated serious adverse events (AEs). In group1: the median time to achieve an EPO CR was 34 days (17-67) after first consolidation and 41 days (12-67) after second consolidation (p=0.35). In group 2: the median time to achieve EPO CR was 39 days (14 - 180). After the pair-matched analysis, 44 patients in each group were matched with at least one case-control patient. When comparing RBC and Pt transfusions, there were 712 units and 751 units in the matched population versus 504 and 669 in the EPO population respectively [208 spared RBC (80 in group1, p=0.008 and 128 in group 2, p=0.004) and 100 spared Pt units (all in group1, p=0.001)]. The multivariate analysis studying different confounding factors on the cumulative incidence of EPO CR showed a significant positive impact of younger age (p=0.001) and intensive chemotherapy (p=0.03) in group1; and for group 2, the positive impact of Pt levels at baseline, the negative impact of female recipient and major ABO incompatibility. We did not find any significant difference in terms of overall (OS) and event free survival (EFS) between EPO and control groups.
This prospective study showed a real benefit of EPO administration on QOL, an achievement of a normal Hb level and a significant spare of RBC and Pt transfusions. Young AML patients, male allo-HSCT recipient, ABO compatible pairs seem to be the best candidates to benefit from EPO administration, with low AEs and no impact on OS or EFS. A cost-effectiveness study is ongoing and results will be communicated.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.