Abstract
Abstract 4144
Adult T-cell leukemia (ATL) is a malignant disorder caused by human T-cell leukemia virus type I (HTLV-I). Morphological discrimination of leukemic cells from non-leukemic T cells is often difficult in ATL since ATL cells reveal morphological diversity except for typical “flower cells”. Although a study using CD3 gating in flow cytometry reported that ATL cells were distinguishable as a CD3low population from normal lymphocytes, these cells were not well characterised as ATL cells. Considering that defective expression of CD7 as well as CD3 is common in ATL cells, we applied multi-color flow cytometry to detect a putative leukemia-specific cell population in the peripheral blood from ATL patients.
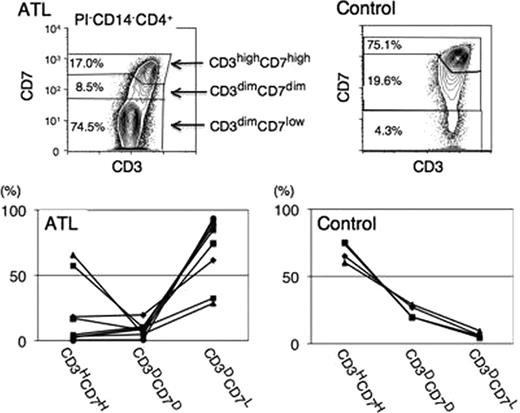
(1) In flow cytometry, after dead-cell and monocyte removal, CD4+ T lymphocytes were gated on the CD3 versus CD4 plot. Based on cell density and fluorescence intensity of CD3 and CD7 in this population, we designated three subpopulations on this plot: CD3highCD7high, CD3dimCD7dim and CD3dimCD7low(Results of a representative ATL and a control sample are shown in Figure). The proportion of the CD3dim/CD7low subpopulation was significantly higher in acute-type ATL CD4+ lymphocytes than in normal controls(Figure). (2) To extensively characterise this subpopulation, we next estimated the HTLV-I proviral load by quantitative real-time PCR after FACS sorting based on this CD3 versus CD7 plot. In all patient samples, HTLV-I proviral integration was detected in all subpopulations. However, the proviral load was significantly higher in the CD3dim/CD7low subpopulation compared to the CD3high/CD7high subpopulation. Almost all of the cells in the CD3dim/CD7low subpopulation were HTLV-I infected. (3) We next examined CCR4 and CD25 expression in each subpopulation. Both CCR4 and CD25 expression levels were maintained at very low and similar levels throughout all subpopulations in normal control cells and in the CD3high/CD7high subpopulation of patients with ATL as well. In contrast, CCR4 expression was significantly up-regulated in CD3dim/CD7low subpopulation of patients with ATL compared to the CD3high/CD7high subpopulation (MFI: 36.5±17.2 vs. 3.8±1.1). The expression of CD25 was also up-regulated in the subpopulation (MFI: 7.8±8.0 vs. 2.7±1.6). (4) Monoclonal expansion of HTLV-I-infected cells in the CD3dim/CD7low subpopulation was indicated by the genomic integration site analysis using a long inverse polymerase chain reaction (PCR) method. (5) We reviewed the glass-slide specimens of FACS-sorted samples to evaluate the morphology of each subpopulation on the CD3 versus CD7 plot. Atypical lymphocytes with morphology such as a notch in the nucleus were observed in all subpopulations. The majority of sorted cells from CD3dim/CD7low subpopulation showed “flower cell”-like morphology. (6) We also detected a small CD3dim/CD7dim subpopulation other than the CD3dim/CD7low and CD3high/CD7high subpopulations in all patients with acute-type ATL who were analysed(Figure). This subpopulation contained the same clone as the CD3dim/CD7low subpopulation, although a phenotypical difference existed between these subpopulations.
(1) Above findings indicate that leukemic T cells are specifically enriched in a unique CD3dim/CD7low subpopulation of CD4+ T cells in acute-type ATL. This multi-color FACS system may be useful for precisely monitoring disease during chemotherapy, detecting minimal residual disease and analysing ATL cells. (2) Previous reports have revealed that HTLV-I-infected cells transform through multi-step oncogenesis. Detailed analysis of these three subpopulations (CD3high/CD7high, CD3dim/CD7dim and CD3dim/CD7low) may give some insight into oncogenesis of HTLV-I-infected cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.