Abstract
Abstract 4227
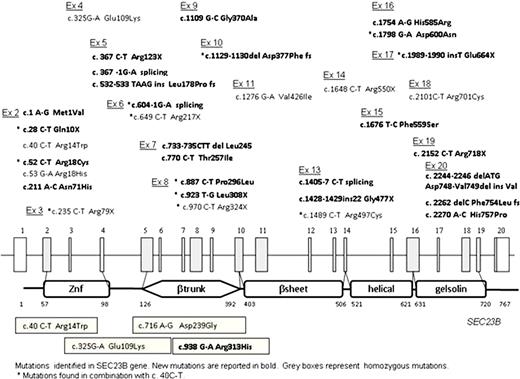
CDAII, the most frequent type of congenital dyserythropoietic anemia, is an autosomal recessive disease characterized by ineffective erythropoiesis, peripheral hemolysis, erythroblast morphological abnormalities and hypoglycosylation of some RBC membrane proteins. Last year we and others identified SEC23B as the gene responsible for CDAII (Schwarz et al, 2009, Bianchi et al, 2009). SEC23B is a member of the SEC23/SEC24 family, a component of COPII coat protein complex which is involved in protein trafficking through membrane vesicles from the endoplasmic reticulum to the Golgi apparatus. The gene, localized on chromosome 20p11, is split in 20 exons and codifies for a 767 aa protein. The aim of the study was to characterize the molecular defect in a large series of CDAII patients of Caucasian origin. Fifty-one CDAII patients from 49 unrelated families (17 Italians, 20 German or Swiss, 4 Dutch, 2 British, 2 Czech and 1 Greek, Turkish, Bulgarian and Irish origin) were analyzed by direct exon sequencing. We identified 36 different mutations, 25 of which were not described before. Eighteen were disruptive and 18 were missense mutations. The patients' molecular data are summarized in the Figure (novel mutations are reported in bold). All the missense mutations affected highly conserved aminoacids and were not found in 200 normal alleles examined. The c.325G>A mutation was identified in 15 homozygous patients and in two cases in combination with other mutations. The change c.40C>T was detected in 16 unrelated patients as heterozygous mutation and, for the first time, at the homozygous level in one patient only. Considering the entire series of patients (including previously published cases) characterized by our groups (86 CDAII patients from 77 unrelated families) c.325G>A and c.40C>T mutations account for 49% (76/154) of the unrelated mutated alleles and therefore should be firstly screened during molecular diagnosis of CDAII. A c.325G>A mutation usually results in a mild to moderate clinical picture at homozygous level, whereas it may cause a very severe clinical pictures when combined with other mutations (Fermo, et al 2010). Despite the sequencing of all exons and flanking intronic regions, 5 patients displayed only one mutation, suggesting the possibility that mutations could be located in regulatory regions or that a second gene could be involved in the pathogenesis of CDAII.
P. Bianchi and K. Schwarz contributed equally to this work.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.