Abstract
Abstract 4279
A fraction of patients (up to 30%) with transfusional iron overload on deferasirox (DFX, Exjade®) have less-than-adequate chelation responses at doses above 30 mg/kg/day. Both transfusion burden and adherence play significant roles in observed chelation effectiveness, but a biological difference between good and poor DFX responders is detectable by pharmacokinetic (PK) assessment. Indeed, we have reported that patients with poor responses at doses>30 mg/kg/d have decreased exposure (area under the concentration:time curve) after a single oral dose of 35 mg/kg (Chirnomas et al, Blood, 2009, 114:4009). In principle, iron status in these individuals could be improved by strategies to reduce iron input or intensify chelation. Here we report results of these strategies for the poor responders of the published PK trial.
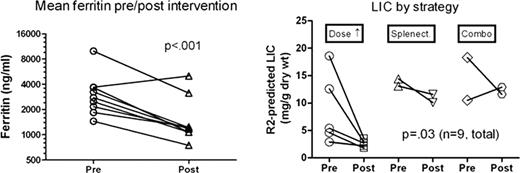
We retrospectively assessed response of ferritin levels and hepatic iron measurements by R2 Ferriscan® MRI to various strategies in the 9 thalassemia (thal) patients (pts) with poor DFX response from the phase 4 PK trial cited above. One sickle cell pt from the trial with documented poor adherence is not included. Strategies were individualized, based on prior dose of DFX, comorbidity (e.g. liver disease or dose-limiting toxicity), transfusion burden, and estimates of pt adherence. Strategies included: splenectomy to reduce transfusion burden (n=2); dose increase (n=5, including 1 with rapid elimination half-life who also received b.i.d. dosing) and combination (“combo”) therapy with DFX and deferoxamine (DFO, n=2). As ferritin values are variable and not normally distributed, log-transformed ferritin values in three successive months from the time of study eligibility determination and after intervention strategies were compared by two-way ANOVA. Liver iron content pre- and post-interventions, was compared by paired t-test.
As shown in the figure, ferritin improved in 8/9 pts (p<.001) and overall, LIC also improved in 8/9 (p=0.03, with variable responses by strategy. In all 5 pts with dose increase [to 35 (n=1) and 40 mg/kg/d (n=4)], ferritin improved significantly. The two splenectomized pts had previously failed to respond to doses of 40 mg/kg, and improved over 3 yrs, continuing 40 mg/kg/d DFX with lower transfusion rates. Two pts switched to combo therapy. One had transaminitis on high dose DFX alone, but improvement on DFX 20 mg/kg/day + DFO (mean daily dose 41 mg/kg/d). The other, with cirrhosis, had inadequate response to DFX 45 mg/kg/day, but improved liver iron on DFO 27 mg/kg/day average plus DFX 40 mg/kg/d). Higher doses of DFX are generally well tolerated, but transaminitis and GI side effects including abdominal pain and diarrhea were observed even in this small cohort. Five of the 9 pts have achieved our target of LIC less than 7 mg/g dry wt, and 8 of 9 have improved iron status overall, while one had worse LIC by MRI
We demonstrate improved chelation in a majority of pts, but not all, in a cohort with a demonstrated biologic contributor (impaired bioavailability) to lower DFX response.
The findings support an approach of individualized therapy to help optimize iron burden. As some of the strategies are not mutually exclusive, they can likely be combined. Doses up to 40 mg/kg/d DFX are now supported in the manufacturer's label. We note that a small fraction of pts does not respond adequately to these augmented maneuvers, and these pts therefore require new approaches.
Chirnomas:Novartis: Research Funding. Off Label Use: Combination therapy with deferasirox and deferoxamine. Paley:Novartis: Employment. Neufeld:Novartis, Inc: Research Funding; Ferrokin, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.