Abstract
Abstract 4414
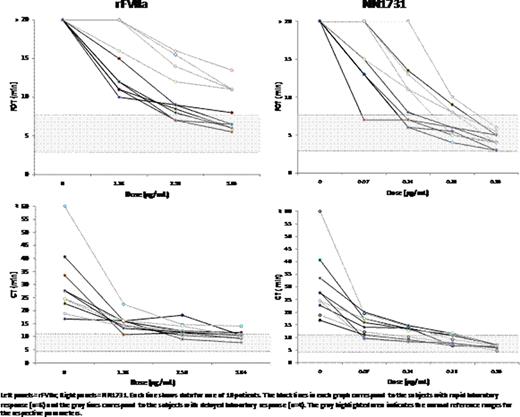
A rFVIIa analogue (NN1731) with increased prothrombinase activity on the activated platelet surface relative to rFVIIa has been shown to result in a more rapid and less variable response in spiking experiments with hemophilia whole blood samples. In a recent pharmacokinetic study of rFVIIa in 10 non-bleeding hemophilia A and B patients who received a dose of 90 mg/kg rFVIIa, we noted that there were two divergent groups based on their laboratory response to rFVIIa. Study participants who achieved clot formation time determined by Hemodyne (FOT) or Rotational Thromboelastography (CT) < 15 min were noted to have a “rapid laboratory response”; conversely, those with a FOT and CT value ≥ 15 min were noted to have a “delayed laboratory response”. In order to determine whether the participants with a delayed laboratory response to rFVIIa 90 μ g/kg would have an improved response to higher doses of rFVIIa and to NN1731, additional blood samples were collected and spiked ex vivo.
Blood samples from ten severe FVIII or FIX deficient patients were spiked with 1.28, 2.56, 3.84 μ g/mL rFVIIa (corresponding to 90, 180 and 270.
Hedner:Novo Nordisk A/S: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Speakers Bureau. Ezban:Novo Nordisk A/S: Employment, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.