Abstract
Abstract 48
BCR signaling is increasingly recognized as a central mechanism for disease progression in CLL. PI3K transmits various external, microenvironment-derived signals that influence survival, drug resistance, and cell migration of CLL cells. PI3K isoforms have distinct functions and tissue distributions. P110δ mutant mice display defective BCR signaling, along with impaired B cell development and differentiation. Because of this unique role of p110δ in BCR signaling, p110δ inhibitors have been developed for treatment of B cell malignancies and autoimmune disorders. CAL-101, a potent and selective p110δ showing an IC50 of 2.5 nM in vitro and >200-fold selectivity relative to other PI3K isoforms, displays pre-clinical and clinical activity in CLL. Prior in vitro CLL studies revealed that CAL-101 induces caspase-dependent apoptosis, and inhibits CD40L-, BAFF-, TNF-alpha- and fibronectin-derived survival signals.
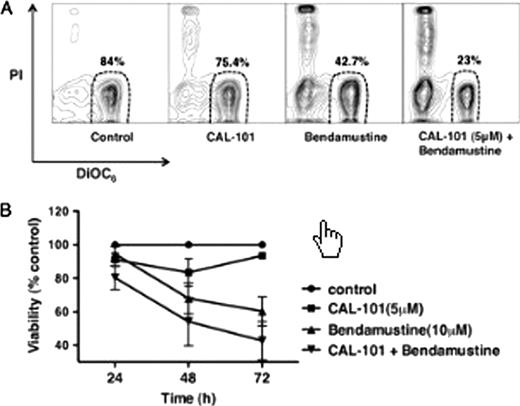
Because of the importance of p110δ in BCR signaling, we tested the effects of CAL-101 on BCR-derived CLL cell activation, and confirmed our findings in correlative studies related to an ongoing CAL-101 phase I clinical trial. We found that BCR cross-linking with anti-IgM significantly increased CLL cell viability to 121 ± 5 % of controls (mean ± SEM, n=15, *P< 0.05).This pro-survival effect was abrogated by CAL-101, which reduced CLL cell viability to 85± 3 % of controls at 48 hours (mean ± SEM, n=15, *P< 0.05). CLL cell viability in co-culture with NLC was also significantly reduced by CAL-101 to 64± 6% of untreated controls at 48 hours (mean ± SEM, n=10, *P< 0.05). BCR cross-linking induces secretion of the chemokines CCL3 and CCL4 by CLL cells, which was quantified in CLL supernatants by ELISA. CAL-101 significantly reduced supernatant CCL3 concentrations from 4060 ± 1392 pg/mL to 2901 ± 1220 pg/mL, and CCL4 levels from 5721 ± 1789 pg/mL to 3223 ± 1311 pg/mL (mean ± SEM, n=6, *P<0.05). In CLL-NLC co-cultures, CAL-101 also inhibited CCL3/4 secretion by CLL cells.Here, the CCL3 concentrations were reduced by CAL-101 from 943 ± 535 pg/mL to 156 ± 8 pg/mL, and the CCL4 concentrations from 7433 ± 4463 pg/mL to 316 ± 53 pg/mL (mean ± SEM, n=5, *P<0.05).CAL-101 also decreased CXCL13 levels in CLL-NLC co-cultures from 151 ± 35 to 70 ± 27 pg/mL (mean ± SEM, n=4, *P=0.05), indicating that CAL-101 has pharmacological actions on both, CLL cells and the CLL microenvironment as represented by the NLC. Given the importance of cytotoxic agents in the therapy of patients with CLL, we evaluated whether a combination of CAL-101 with bendamustine, could overcome stroma-mediated drug resistance in CLL cells co-cultures with marrow stromal cells (MSC). Fig A shows contour plots of a representative case that depict CLL cell viability after treatment with CAL-101, bendamustine, or the two drugs combined. Fig. B displays a bar diagram that depicts the mean relative viabilities of CLL cells treated with CAL-101 (5 mM),bendamustine(10 mM),or the drug combination (mean ± SEM, n=4). The consistently higher CLL cell death of CLL cells treated with the combination of both drugs indicates an additive effect.
Next, we used phospho-flow to demonstrate that CAL-101 inhibits constitutive and BCR-induced PI3K pathway activation in samples obtained from CLL patients undergoing treatment with CAL-101. CAL-101 treatment down regulated pAkt(T308) in peripheral CLL cells by >90% (n=12). Plasma samples from 14 CLL patients obtained before and after 28 days of daily treatment with CAL-101 were analyzed for concentrations of various cytokines. Interestingly, these analyses revealed substantial decreases from baseline to Day28+ of CAL-101 treatment in mean plasma levels of CCL3 (from 186 pg/mL to 29 pg/mL), CCL4 (from 303 to 70 pg/mL),CCL22 (1067 to 533 pg/mL), CXCL13 (316 pg/mL to 40 pg/mL), and TNFα(104 to 29 pg/mL), confirming our in vitro data related to CCL3/4 and CXCL13.
Collectively, our data demonstrate that CAL-101 effectively inhibits BCR- and NLC-mediated CLL cell survival and activation in vitro. Also, CAL-101 enhances the activity of cytotoxic agents such as bendamustine against CLL cells. Our in vivo data indicate that CAL-101 decreases elevated pre-treatment CCL3 and CCL4 levels. These findings are consistent with the concept that inhibition of BCR-derived signals could be a key mechanism of action of CAL-101 in CLL. The results also support clinical evaluation of CAL-101 in combination with bendamustine for the treatment of CLL.
Meadows:Calistoga Pharmaceuticals: Employment. Lannutti:Calistoga Pharmaceutical Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.