Abstract
Abstract 4970
While the epidemiologic and demographic characteristics of veterans with MDS have been established (Komrokji et. al, 2009), there is no data on prognostic factors. Several factors including age (Kim SH et. al, 2010), sex (Nosslinger et al 2010), ferritin (Armand et. al 2007), MCV (Wang et. al 2010), use of growth factors (Jaderstan et. al 2008), and co-morbidities (Sperr et. al 2010) independent of the established WHO prognostic scoring system (WPSS) (Malcovati et. al 2007) have been shown to impact survival in MDS. The purpose of this study is to highlight major survival determinants among veterans with an emphasis on transfusion dependency and ferritin.
Charts of 88 unselected patients (87 males) with a pathological diagnosis of MDS from January 2000 to December 2008 at the Michael E. Debakey VA Medical Center in Houston, Texas were included. The following data was collected at diagnosis: demographics, smoking, alcohol use, initial ECOG performance status, hematological data, cytogenetics, HIV and hepatitis status. Transfusion dependency was defined as needing more than 1 unit of packed red cells each month for 4 consecutive months at anytime after diagnosis. 50 patients had 4 or more ferritin readings spread over a minimum of 4 months, to compute a meaningful mean increase in ferritin per year (MIF). Administration of growth factors, treatment with Azacytidine, Decitabine or Lenalidomide was analyzed as categorical data (intervention or no intervention).
Univariate survival analysis was performed using GraphPad Prism 5.0, and Kaplan-Meier curves were computed. Multivariate analysis on significant variables was performed using the Cox model on MedCalc Statistics Software (version 11.3).
Demographic factors: The median age at diagnosis was 73 (53-91) with a median OS of 520 days (13-2234). 9 patients (10.2%) progressed to AML at a median time to progression of 159 days (74-614), all with IPSS scores > 1 at diagnosis. Distribution [N (Median OS)] among WHO sub-groups were as follows: RA: 22(659), RARS: 10(1020), RCMD: 39(638), RAEB-1: 5(228), RAEB-2: 9(170), 5q-: 3(521). Age, race, tobacco, alcohol use, hepatitis, HIV status were not significant prognostic variables. Performance status (ECOG) > 2 (p=0.012), and blood group O were the only significant variables on univariate analysis (p=0.029).
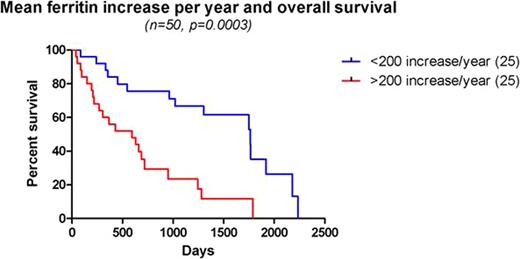
Initial Hb < 8 g/dl, IPSS(Greenberg et. al, 1997) scores > 1.0, transfusion dependency, cytogenetic abnormalities, MIF > 200 per year, MCV < 100 and use of erythropoetin were significant variables in predicting OS on univariable analysis. On multivariate analysis, IPSS scores > 1.0 (p=0.01), transfusion dependency (p=0.0029), ECOG performance status > 2 (p=0.0421) and MIF > 200 (p=0.0294) were independent significant prognostic factors. In the sub-group of MDS without excess blasts (Cermak et. al, 2009), transfusion dependency and MIF > 200 per year were highly significant variables (p<0.0001). Use of Azacitidine (8), decitabine (8) or lenalidomide (4) did not impact survival (p=0.63), although only 14 patients overall received one or more of these treatments.
Veteran's with MDS pose a unique population, being predominantly male, older age at diagnosis and with potentially poorer performance status. Based on the above results, these patients had a poorer median survival (1.42 years) than expected from established data (2.1 years, Komrokji et. al 2009). The established prognostic factors, the IPSS scores and transfusion dependency expectedly were independent predictors of OS. Iron overload is rapidly becoming a leading problem in the management of MDS. This is most evident in the transfusion dependent MDS patient without excess blasts with better prognosis and longer median survival, for iron overload to manifest its deleterious effects. MIF values > 200 were intended to represent populations at higher risk for developing iron overload. Interestingly, MIF was independent of transfusion dependency in predicting OS. This highlights the need for prospective studies to establish the role of ferritin and its rise over time to identify populations at risk for iron overload and mortality thereof. The clinical question of benefit from chelation therapy in these patients remains.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.