Abstract
Abstract 52
The T cell leukemia/lymphoma 1 (TCL1) oncogene was initially identified as a target of chromosomal translocations and inversions at the 14q32.1 chromosome breakpoint region in T-cell prolymphocytic leukemia (T-PLL). Deregulation of TCL1 in mouse B cells results in a CD5+ B cell leukemia that mimics aggressive human chronic lymphocytic leukemia (CLL), indicating that TCL1 plays an important role in the pathogenesis of CLL. However, chromosomal aberrations that constitutively activate TCL1 have not been identified in CLL, and the mechanism(s) of TCL1 activation in CLL remain unclear.
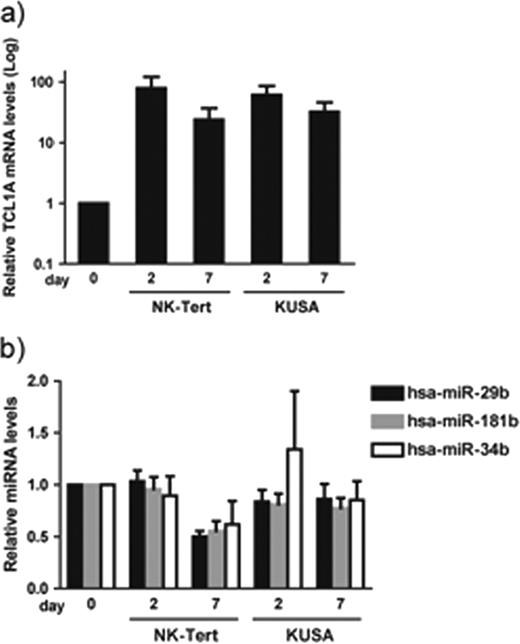
We co-cultured CLL cells with two marrow stromal cell (MSC) lines (KUSA-H1, NK-Tert) and found TCL1 to be among the top genes up regulated after co-cultures with MSC. This was based on at least 3-fold up-regulation in paired samples, using Affymetrix HG U133 plus 2.0 oligonucleotide arrays. We also found an up-regulation of TCL1 mRNA by qRT-PCR and TCL1 protein by immunoblotting and flow cytometry. qRT-PCR revealed significant increases in TCL1A mRNA levels with up to 100 fold increases after 2 and 7 days of co-culture in an independent set of 8 CLL samples (Fig. a). Because TCL1 is a co-activator of the AKT oncoprotein and its downstream target MCL-1, we next explored AKT and MCL-1 activation after MSC co-culture by immunoblotting. We found increases in TCL1A protein to be associated with robust increases of pAKT, AKT isoforms 1 and 2, and MCL-1 after MSC co-culture. In order to investigate the possible mechanism of regulation of TCL1 expression by MSC, we next examined the levels of TCL1 negative regulators miR-29b, miR-181b and miR34b targeting the 3’ UTR of TCL1 (Pekarsky et al., Cancer Res. 66:11590-3, 2006). qRT-PCR revealed decreased levels of miR-29b, miR-181b, and miR-34b after 7 days of co-culture with MSC (Fig. b), which paralleled the increased levels of TCL1. This inverse correlation was more marked in some cases than in others, and this heterogeneity did not correlate with the initial miRNA or TCL1A levels. The timing of these events suggests, that miRNA based modulation of TCL1 levels is only in part responsible for TCL1 up regulation in MSC co-cultures. It rather implicates multiple mechanisms to be responsible for milieu mediated TCL1 regulation, i.e. transcriptional and/or post-transcriptional. The fact that all analyzed miRNAs are simultaneously down regulated in our system also suggests a common superimposed regulatory mechanism in miRNA processing/regulation that might contribute to both direct and miRNA mediated TCL1 up regulation in MSC co-cultures. In summary, our results indicate that MSC induce TCL1 expression uniformly in primary CLL; the induction of TCL1 is associated with downregulation of miR-29b, miR-181b, and miR34b, along with induction of pAKT, AKT 1/2, and MCL-1. Collectively, these findings suggest that the microenvironment plays a proactive role in regulation of TCL1 expression.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.