Abstract
Abstract 54
B-cell chronic lymphocytic leukemia (CLL) is characterized by the progressive accumulation of CD5+ B-lymphocytes in the peripheral blood, lymphoid organs and bone marrow. Despite aggressive therapy, CLL is still incurable partly because of intrinsic defect in apoptosis induction. Deregulated expression of protein kinases by gene deletion, mutation, or amplification has been found to be important for tumor initiation and progression involving cancer cell proliferation, survival, motility, and invasiveness as well as tumor angiogenesis and chemotherapy resistance. Because of their critical functions in oncogenesis, protein kinases have been at the forefront of targeted cancer therapy development since the 1980s. A novel receptor tyrosine kinase (RTK) Axl has been reported to be overexpressed in several types of human cancers including colon, prostatic, thyroid, breast, gastric, renal and lung. Indeed, our recent investigation (ASH 2009, Abstract #2368) identified Axl to be constitutively phosphorylated in CLL B-cells obtained from a majority of CLL patients. We wished to examine whether the constitutively phosphorylated Axl plays a pivotal role in regulating leukemic CLL B-cell survival and could be a potential target to treat this disease.
We used freshly isolated purified CLL B-cells after obtaining informed written consent from the patients. Expression status of various kinases in relation to Axl expression and their functional involvement with this novel RTK to drive downstream cell survival signaling pathway(s) were analyzed in CLL B-cell lysates by immunoprecipitation/Western blot analyses using specific antibodies. Finally, we evaluated the functional importance of Axl in CLL by employing two RTK inhibitory agents (Bosutinib [SKI-606] and R428) of different specificity and affinity for receptor tyrosine kinases in order to target Axl and determined the impact on apoptotic/cytotoxic effects on CLL B-cells by flow cytometric analysis. In some experiments, we used bone marrow stromal cells to evaluate if they could diminish the impact of the RTK agent R428.
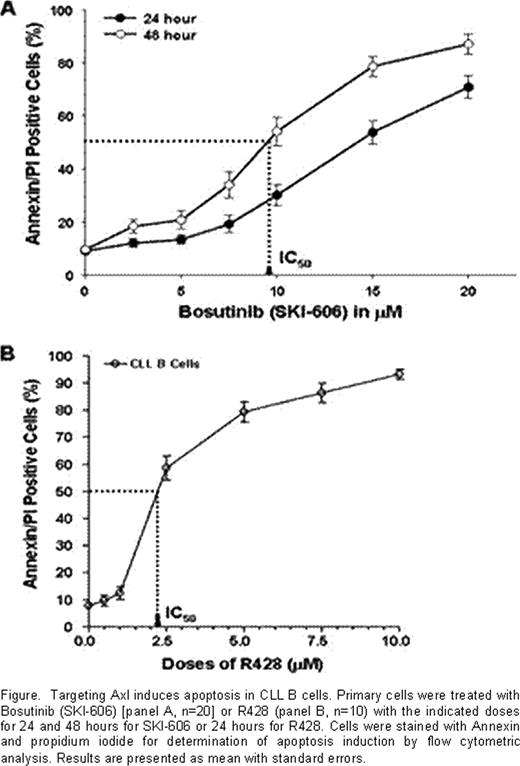
To further our initial examination of Axl status in CLL, we assessed the expression of phosphorylated-Axl (P-Axl) in freshly isolated CLL B-cells by Western blot analysis. Indeed, we detected differential levels of P-Axl in CLL B-cells (n=10). Further analysis revealed that the expression of P-Axl was associated with co-expression of other constitutively phosphorylated kinases/enzymes including Src family kinases (SFKs), PI3K, SyK/ZAP70 and PLC-g2 in CLL B-cells. Importantly, we found by immunoprecipitation of Axl and subsequent Western blotting that these intracellular signaling molecules were complexed with P-Axl in primary CLL B-cells. Further analysis using a siRNA approach targeting Axl suggests that phosphorylation of Axl is an upstream event of SFK-activation in CLL B-cells indicating a pivotal role of Axl in regulating CLL B-cell survival. Finally, when Axl and Src kinases were targeted by a Src/Abl kinase inhibitor, SKI-606 (dose range 2.5–20mM) or the specific-inhibitor of Axl, R428 (dose range 0.5–10 mM) robust induction of CLL B-cell apoptosis was observed in both a dose- and time-dependent manner (Figure). Although both RTK inhibitory agents showed clear induction of apoptosis in CLL B-cells, R428 was found to be the most potent in inducing apoptosis exhibiting an IC50 ∼4-fold lower than SKI-606.
Furthermore, we have observed that R428 could target P-Axl resulting in inhibition of SFK-activation further suggesting that Axl regulates SFK-activity in CLL B-cells. Interestingly, R428-induced apoptosis in CLL B-cells (doses of 1.0 mM and 2.5 mM) was significantly inhibited when these leukemic cells were co-cultured with bone marrow stromal cells, but when we tested a higher dose (5 mM) of R428 this stroma-mediated protection was overcome. Further studies are underway to test R428 in combination other chemotherapeutic agents.
Thus, we have identified a novel RTK, Axl, in CLL B-cells which appears to work as a docking site for multiple non-RTKs and drives cell survival signals. These findings indicate a unique therapeutic target for CLL treatment. Importantly, there are currently several Axl inhibitory agents that are available to be tested in CLL patients.
Holland:Rigel: Employment.
Author notes
Asterisk with author names denotes non-ASH members.