Abstract
Abstract 544
Conventional dosing of FVIII prophylaxis in adults with hemophilia is based on 3 weekly or alternate day regimes of 20–40IU/kg, aiming for a trough level above 1IU/dL. However, pharmacokinetics of FVIII widely varies between individuals, thus the amount of factor concentrate required to maintain trough levels above 1IU/dL is also disparate. Mathematical modeling indicates that half-life has a significant influence over trough level. An individualized dose based on FVIII half-life may therefore allow for more appropriate dosing of prophylaxis in adults. For this approach to be effective, half-life results obtained from pharmacokinetic studies need to be consistent and representative of an individual's steady state. Although it is recognised that half-life increases from early childhood until adolescence, intra-individual variation of half-life in adults has not been examined.
We hypothesized that for patients >18years significant inter-individual variation, but minimal intra-individual variation, in half-life would be detected.
This single center study investigated inter- and intra-individual variation in FVIII half-life based on 140 pharmacokinetic studies conducted in 73 individuals with severe hemophilia A (FVIII:C < 1IU/dL). Data for all FVIII pharmacokinetic studies performed over a 5-year period were extracted from an in-house database. Patient demographics and laboratory results were verified against electronic patient records. All half-life studies were performed according to a set protocol and analyzed within the same clinical pathology accredited reference laboratory. Results were tested using the non-parametric Wilcoxin signed rank test. Data is presented as median and range and p-values < 0.001 were considered significant.
The median age of patients was 17.5 years (range 5.0– 54.0).
The wide range in FVIII half-life data indicated substantial inter-individual variation within the complete population (median 9.9hrs, range 2.3–19hrs), replicated upon division of data into age specific categories (Fig. 1).
36 patients had undergone multiple studies over the 5-year period. Of these, 21 had at least 3 data sets. FVIII half-life showed considerable intra-individual variation with 17 patients (81%) demonstrating a range of half-life greater than 2hrs.
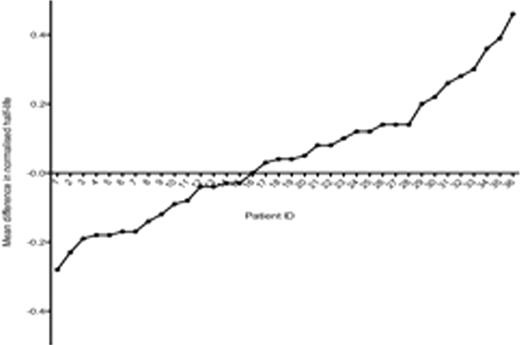
FVIII half-life data was normalized to nullify the effect of extensive inter-individual variation in half-life within the population. Figure 2 demonstrates mean difference in normalized FVIII half-life for all patients with multiple half-life results. Testing against a null hypothesis of no variation, we found significant intra-individual variation upon examination of the entire population (n= 36, p<0.0001). Following exclusion of data from children less than 6 years, significant intra-individual variation could still be detected for children 6–18 years (n=19, p< 0.0001), and adults (n= 16, p<0.0001).
This study verifies expected inter-individual variation of pharmacokinetic profiles and appears to support the concept of individualized prophylaxis regimes in adults based on half-life data. However, considerable intra-individual variation in FVIII half-life in adult patients was registered. Mathematical models have indicated that even modest changes in half-life influence dosing requirements for FVIII prophylaxis. Intra-individual fluctuations in FVIII half-life will necessitate regular pharmacokinetic studies to ensure appropriate dosing. This will naturally affect the cost, utility and acceptability of treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.