Abstract
Abstract 602
T–cell depleting regimens that contain anti-thymocyte globulin (ATG) have been used for the treatment of myelodysplastic syndrome (MDS) suggesting a link between immune dysregulation and ineffective hematopoiesis in a subset of patients. Pre-treatment clinical features such as younger age, shorter duration of red cell transfusion dependence, and HLADR15 genotype were identified as response co-variates in a single institutional experience with equine ATG (eATG). We report results from the first US multicenter study examining rabbit (rATG) for the treatment of MDS.
This study was a non-randomized, multicenter Phase II clinical trial of rATG (Thymoglobulin®) designed to examine the response and safety of this agent in MDS patients. The primary endpoint was IWG 2000 response rate with secondary analyses of duration of response, time to response, time to progression and tolerance. rATG 2.5 mg/kg/day was administered IV for 4 doses. The relationship between response and variables such as age, bone marrow cellularity, HLA-DR15, karyotype, blood counts, and disease duration were also investigated. Eligible patients had severe neutropenia (ANC < 1000), severe anemia (untransfused hemoglobin <9 g/dl), anemia requiring transfusion, or thrombocytopenia (platelet count < 50,000/mm3) plus the presence of 10% bone marrow blasts in patients with int-2. Patients with chronic myelomonocytic leukemia (CMML) and lab abnormalities were excluded. Statistical methods include Wilcoxon rank-sum test, Kaplan-Meier survival curve and log-rank test.
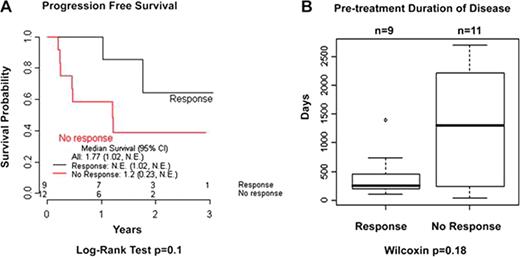
Between Aug.2004 and May 2010, 39 MDS patients were enrolled: 24 patients were eligible for treatment, and 21 evaluable for response. Drop out occurred due to initiation of therapy off study (n=5), non-compliance (n=1), death prior to treatment (n=2), and failure to meet eligibility criteria (n=7). IPSS risk categories in the evaluable patients included low-risk (n=6, 29%), int-1 (n=12, 57%), and int-2 (n=3, 14%). Infusion-related reactions were the most common adverse event, generally occurring with the first dose of rATG. There were 3 deaths among treated patients related to infection, including 1 patient with neutrophil hematologic improvement (HIN). Nine (43%) of the 21 evaluable patients experienced HI. Twelve (57%) were non-responsive including patients with stable disease (n=7, 33%) and disease progression (n=5, 24%) including 3 with excess blasts (n=2) or prior history of cancer treatment (n=1). The median time to response was 75 days (range 3 days–3.7 months) and median duration of response was 7.2 months (range 2–22+ months) with a median follow up of 20 months. Two patients maintained HI at the time of this analysis and response was extended in two patients by initiating cyclosporine As shown in Fig. 1, progression free survival (PFS) was greater in responders compared to non-responders (median PFS in responders 1 vs 0.23 year), but did not reach statistical significance (p=0.1). The mean age at treatment for evaluable patients was 64 years (median 66, range 44–79), however, age was not a significant co-variate for HI (median age in responders 65 years, non-responders 66, p=0.5). Only 4 patients had the HLADR15 allele, among whom 3 achieved HI (75%). Other factors such as IPSS, cytopenias, LDH, karyotype, age-adjusted bone marrow cellularity, and M:E ratio showed no association with HI in univariant analyses. Responding patients had a shorter time from diagnosis to treatment (responders median 8 months vs non-responders median 41.8 months, (p=0.18).
This multicenter study provides evidence that rATG is safe and has significant activity in MDS that warrants further study. Although independent of age, a pretreatment rATG response algorithm inclusive of HLADR15 and duration of disease appears to be similar to prior predictive models of eATG response. These results suggest that T-cell depleting rATG therapy may produce the best clinical outcome when implemented early in the disease process and further suggests that T-cell hematological suppression may contribute to the initiation of MDS pathogenesis.
This trial was conducted by the Bone Marrow Failure-Rare Disease Clinical Research Network (BMF-RDCRN 5406) sponsored by the NIH and Genzyme, Corp. This trial was registered at www.clinicaltrials.gov (NCT00466843).
Epling-Burnette:Genzyme: Consultancy, Research Funding. Maciejewski:Genzyme Corp: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.