Abstract
Abstract 664
Soluble CD40 ligand (sCD40L) rapidly translocates to the surface of activated platelets and plays a role in the inflammatory cascade. sCD40L has been shown to not only accumulate during the storage period of platelets, but has also been linked with TRALI and febrile reactions following platelet transfusions. Licensed plasma has significant platelet contamination which introduces platelet-derived inflammatory proteins into the plasma. Blood components containing greater than 30 ml of plasma have the highest rate of transfusion-associated complications, most notably TRALI. Both centrifugation at 12,500g as well as leukofiltration are capable of decreasing platelet levels in plasma by 2 logs. We hypothesize that pre-storage platelet reduction will decrease the accumulation of sCD40L in licensed plasma, which will reduce the pro-inflammatory potential of thawed plasma, and abate neutrophil (PMN) priming in vitro.
Plasma preparation. Units of whole blood were collected from healthy human donors. Control units were prepared in accordance with AABB standards. For units undergoing cell depletion centrifugation (CDC), following the primary separation from the red cells, the plasma was centrifuged at an additional 12,500g for 7 minutes at 4°C. For leukofiltration, whole blood was filtered using Pall Corporation (Port Washington, NY), MacoPharma (Mouvaux, France), and Terumo (Elkton, MD) filters per the manufacturers' specifications. The plasma was then separated and frozen per AABB standards. Plasma samples were thawed in a 37°C shaking water bath and then stored at 4°C for 5 days. Levels of sCD40L were measured on days 1, 3, and 5 post-thaw using a commercial ELISA kit (R&D Systems). PMN isolation & priming. Heparinized whole blood was drawn from healthy human donors, and PMNs were then isolated by standard techniques: dextran sedimentation, Ficoll-Hypaque gradient centrifugation, and hypotonic lysis. PMNs were incubated with 10% plasma at 37°C for 5 minutes and then activated with 1 μM formyl-Met-Leu-Phe (fMLP), and the rate of O2− production was measured via the reduction of cytochrome c at 550 nm. Priming activity is the augmentation of the fMLP-activated respiratory burst (nmol O2−/min).
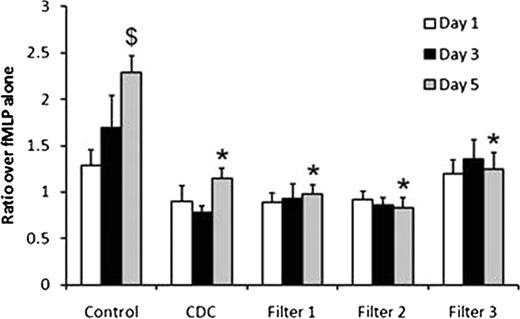
Control plasma showed a significant increase in sCD40L concentration and PMN priming activity over the 5 day storage period (Figure 1). Both CDC and pre-storage whole blood leukofiltration decreased sCD40L concentration vs. controls (Table 1). There were no significant increases in sCD40L in leukofiltered plasma over 5 days of storage. There was no increased priming activity over the 5 days of storage in CDC and leukofiltered units.
The data are expressed as the mean±SEM fold over fMLP alone (n=8). *Decreased from control plasma on the same day; $Increased when compared to Day 1 of preparation (p<0.05).
The data are expressed as the mean±SEM fold over fMLP alone (n=8). *Decreased from control plasma on the same day; $Increased when compared to Day 1 of preparation (p<0.05).
The data are expressed as mean±SEM concentration of sCD40L (pg/ml) (n=8).
| . | Day 1 . | Day 3 . | Day 5 . |
|---|---|---|---|
| Control | 190.2 ± 49.7 | 378.7 ± 51.1$ | 311.2 ± 36.5$ |
| CDC | 37.9 ± 11.2* | 105.7 ± 13.2$* | 98.6 ± 9.3$* |
| Filter 1 | 36.5 ± 10.4* | 17.5 ± 5.7* | 32.1 ± 10.9* |
| Filter 2 | 97.0 ± 20.1* | 92.7 ± 20.6* | 73.0 ± 14.8* |
| Filter 3 | 172.3 ± 27.9 | 171.6 ± 29.9* | 171.8 ± 32.3* |
| . | Day 1 . | Day 3 . | Day 5 . |
|---|---|---|---|
| Control | 190.2 ± 49.7 | 378.7 ± 51.1$ | 311.2 ± 36.5$ |
| CDC | 37.9 ± 11.2* | 105.7 ± 13.2$* | 98.6 ± 9.3$* |
| Filter 1 | 36.5 ± 10.4* | 17.5 ± 5.7* | 32.1 ± 10.9* |
| Filter 2 | 97.0 ± 20.1* | 92.7 ± 20.6* | 73.0 ± 14.8* |
| Filter 3 | 172.3 ± 27.9 | 171.6 ± 29.9* | 171.8 ± 32.3* |
Decreased from control plasma on the same storage day;
Increased when compared to Day 1 of preparation (p<0.05).
sCD40L plays an important role in inflammation and its inhibition decreases sepsis-related acute lung injury (ALI) in animal models. Platelet contamination and resultant high levels of sCD40L are associated with increased PMN priming activity in vitro over the storage period of thawed plasma and may be contributing to TRALI risk. The reduction of platelet contamination in frozen plasma may be a valuable TRALI mitigation strategy in the production of licensed plasma.
Bercovitz:National Blood Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.