Abstract
Abstract 722
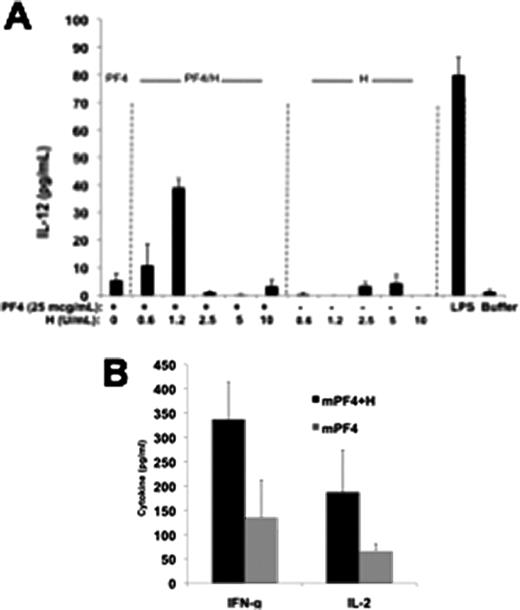
Heparin-Induced Thrombocytopenia (HIT) is caused by antibodies to multimolecular complexes of Platelet Factor 4 (PF4) and heparin (H). Little is known about the cellular mechanisms underlying the PF4/H immune response. Our previous studies have shown that mice injected with murine (m) PF4/H complexes develop a de novo immune response to mPF4/H, but do not respond to injections of mPF4 alone or H alone. In other studies using this model, we have shown that the HIT immune response is T-cell dependent, requires the presence of PF4/H multimolecular complexes and does not engage TLRs via MyD88. To examine the cellular basis of the HIT immune response, we performed studies addressing requirements for antigen presentation. We first isolated splenocytes from non-immunized C57Bl/6 mice and incubated 4×105 splenocytes with mPF4 alone (10mg/ml, final concentration.), heparin alone (0.4U/ml, final), mPF4/H complexes (10mg/ml and 0.4U/ml, final), buffer or LPS (1 mg/ml, final, positive control). We noted significant levels of IL-12 in wells incubated 24hrs with mPF4/H (109 ± 7 pg/mL) or LPS (256 ± 22 pg/mL) but not wells containing mPF4 (33 ± 7 pg/mL), H (5 ± 7 pg/mL), or buffer (9 ± 13 pg/mL). In other studies, we noted that splenic dendritic cells (DCs) were primarily activated by mPF4/H complexes and that cellular activation, as gauged by IL-12 (Figure 1A) or IFN-g (data not shown) occurred in a heparin-dependent manner. DCs activation by mPF4/H complexes was not dependent on CXCR3 or pattern recognition receptors, such as receptor for dectin, mannose or complement receptor 3 (CR3 or CD11b antibody). To determine the effect of DC activation on T-cell responses, we performed mixed lymphocyte reaction assays using DCs isolated from C57Bl/6 mice (3×105 cell/well) and naïve T-cells from Balb/c mice (1:5 cellular ratio). Pre-incubation of Bl/6 DCs with mPF4/H complexes, but not mPF4, H or buffer alone resulted in Balb/c T cell activation and release of cytokines indicative of T helper (Th) 1 immune response (Figure 1B). In summary, we show that DC s are directly activated by PF4/H multimolecular complexes, and that cellular activation by complexes results in a predominant Th1 polarization. Additional studies are underway to identify the relevant receptor for DC activation, and additional pathways of antigen processing and presentation that are necessary for the initiation of a PF4/H specific adaptive immune response.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.