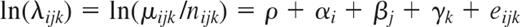The incidence of chronic lymphocytic leukemia (CLL) in Taiwan is markedly lower than that in Western countries, but we have seen a drastically increasing trend. We explored this distinct incidence trend of CLL for Taiwanese. The epidemiologic data of CLL for Taiwanese and Caucasian Americans during 1986 to 2005 were obtained from the Taiwan National Cancer Registry and Surveillance, Epidemiology, and End Results Program, respectively. The individual effects of time period and birth cohort on the incidence trends were analyzed using an age-period-cohort model. Although there was a weak period effect corresponding to the increased applications of immunophenotyping in 1991 to 1995 in Taiwan, evidences suggested that the age-adjusted incidence rate of CLL for Taiwanese was continuously increasing during the 20-year period while that for Caucasian Americans remained steady. In addition, a much stronger birth-cohort effect was identified for Taiwanese but not for Caucasian Americans. This effect corresponded to the westernization of lifestyle in Taiwan since 1960. We conclude that, in addition to the ethnic difference of incidence, there is distinct increasing incidence trend of CLL in Taiwan. The strong birth-cohort effect underlying this increasing trend indicates that lifestyles and environmental factors may play a role in the development of CLL for Taiwanese.
Introduction
Chronic lymphocytic leukemia (CLL) is a clonal lymphoid neoplasm characterized by proliferation and accumulation of neoplastic B lymphocytes in the blood, bone marrow, lymph nodes, and/or spleen.1 In Western countries, CLL is the most common leukemia in adults and accounts for 5%-11% of non-Hodgkin lymphomas (NHLs). The incidence rate is approximately 2 to 6 cases per 100 000 person-years with an increasing trend as people get older.1,–3 However, CLL is much less prevalent in Eastern countries, including Taiwan.4,–6 In these countries, CLL accounts for only 1% to 3% of NHLs in most series.3,7,8 Nonetheless, in our previous report concerning the epidemiology of multiple myeloma (MM) in Taiwan, the age-adjusted incidence of CLL, which was also studied with that of MM, was shown to increase approximately 3.3-fold from 1979 to 2003 in Taiwan.9 Such an increasing trend was not found in Western countries, where the incidence rates of CLL remained relatively stable over time.5 The reasons for the drastic differences in incidences and trends between geographic regions are unclear but are of considerable interest.
The age-period-cohort (APC) model is frequently applied to analyze trends of disease incidences for hypothesis generation.10,11 The age effect usually reflects physiologic differences associated with the susceptibility to a disease among different age groups. The time period effect usually reflects factors that equally affect all age groups at a given period of time, such as the introduction of new diagnostic techniques, implementation of new screening programs, or improvement in completeness of data registration. The birth cohort represents a population born into a specific generation; if disease incidence is affected by the birth cohort, there is a birth cohort effect. The cohort effect involves factors that may have different exposure levels in different birth cohorts. A typical factor that is likely to be reflected by the cohort effect is alternation of lifestyle, which is usually determined early in adulthood.12,13 The birth cohort effect is, therefore, of importance in understanding the implications of lifestyle in the trend of disease incidence. The APC model is able to dissect and estimate the effect of patients' age as well as the individual effects of time periods at diagnosis and patients' birth cohort, which are often overlooked in cross-sectional epidemiologic studies.
At present, available data dealing with descriptive epidemiology of CLL are mainly from Western countries. Few studies have addressed the differences in the incidence trends between Western and Eastern countries. APC modeling has been used in the analysis of various cancers but it has rarely been used to analyze the trends in incidences of CLL.12,13 The current study compared the epidemiologic data of CLL from 1986 to 2005 between Taiwanese and Caucasian Americans with the application of APC model to formulate possible new hypotheses and to elucidate the mechanism for the inter-racial differences in the incidence of CLL.
Methods
Source of data
Epidemiologic data on CLL (ICD-O M-9823/3), including incidence rates, patient numbers, and population sizes, for the period 1986 to 2005 for Taiwanese were obtained from the Taiwan National Cancer Registry (TNCR), a population-based cancer registry founded in 1979 by the Department of Health of Taiwan. TNCR collects information of newly diagnosed cancer patients in hospitals with 50 or more beds. The registry is estimated to encompass approximately 80% of all cancer cases in Taiwan.9,12,–14 Epidemiology data on CLL for Caucasian Americans during the same period were obtained from the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute of the United States. The selection criteria were: SEER registry, 9 SEER registries; site, chronic lymphocytic leukemia; race, non-Hispanic white; and sex, male and female. Data on patients aged younger than 30 years were omitted in both populations because of low case numbers.
Data categorization and plotting
The data were categorized into eleven 5-year age groups (from 30-34 to > 80) and four 5-year time period groups (from 1986-1990 to 2001-2005), implying 14 overlapping 9-year birth cohort groups (from 1902-1910 to 1967-1975). Age-specific incidences were plotted by calendar periods at diagnosis and by birth cohorts for both populations.
Estimation of average annual percentage change
A simple log-linear model was used to estimate the average annual percentage change (AAPC) of incidences to confirm the analytic observations through visual inspection, based on the assumption that, throughout the whole period, an annual growth rate in incidence is constant. A second order polynomial model with a quadratic term was used to detect possible non-linear trends in the establishment of the model.10,11
APC model
The APC model was fitted based on 2 assumptions. First, the number of cases over a time period followed the Poisson distribution. Second, the logarithm of the incidence rate was a multiplicative function of age, time period, and birth cohort. It was expressed as a log-linear model, that is to say a linear combination of the effects of age, time period, and birth cohort as follows:
where λijk, μijk, and nijk denote the incidence rate, the mean number of patients, and the number of individuals, respectively, in the ith age group (i = 1,2,…, I), the jth time period group (j = 1,2,…, J), and the kth birth cohort group (k = I − i + j). αi, βj, and γk represent the effects of the ith age group, the jth time period group, and the kth birth cohort group, respectively; ρ is the intercept term and eijk is the random error term that follows the normal distribution with mean 0 and constant variance. Because of the linear dependence between age, time period, and birth cohort (age = time period − birth cohort), the individual estimate of the effect of the 3 factors cannot be uniquely identified. Therefore, the regression coefficient of the first period effect was constrained as zero to provide first-order relative risk estimates of the period effect. Similarly, the regression coefficient of the first cohort effect was constrained as zero to provide estimates of the cohort effect. Relative risk estimates, reflecting the individual effect of time period and birth cohort, were generated by the maximum likelihood method. The time period 1991-1995 and the birth cohort 1927-1935 were used as reference groups for estimates of relative risk. The degree of freedom and deviance derived from the model were used to measure the goodness-of-fit of each model. A smaller deviance implies a better fit of a given model in the model establishment process. F test was used to test for significant differences in deviance between the age-alone model and the 2-factor models. The analysis was conducted with R software.
Results
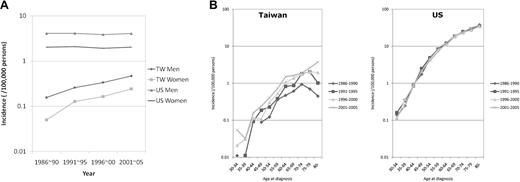
Increasing CLL incidence with a shifting age-specific pattern for Taiwanese
From 1986 to 2005, 931 Taiwanese (643 men and 288 women) were diagnosed as having CLL, while 29 870 Caucasian Americans (17 690 men and 12 180 women) were diagnosed as having CLL. As shown in Figure 1A, the average incidence of CLL in Taiwanese increased from 0.16 (1986-1990) to 0.47 (2001-2005) in men and from 0.05 (1986-1990) to 0.24 (2001-2005) in women. In Caucasian Americans, the incidence remained steady in both sexes (between 3.86 and 4.12 in men and between 1.92 and 2.08 in women). The age-specific incidence rates of CLL in Taiwanese were much lower than those in Caucasian Americans in every age group (Figure 1B). In addition, the peak incidence for Taiwanese was shifting from 70 to 74 years during the period 1986 to 1990 to > 80 years during the period 2001 to 2005, an event that was changing toward the pattern of Caucasian Americans.
Incidence rates. Average age-adjusted incidence rates (A) and age-specific incidence rates (B) of CLL in Taiwanese (TW) and Caucasian Americans (US) 1986 to 2005.
Incidence rates. Average age-adjusted incidence rates (A) and age-specific incidence rates (B) of CLL in Taiwanese (TW) and Caucasian Americans (US) 1986 to 2005.
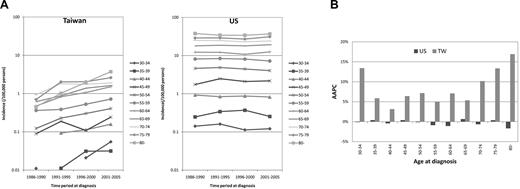
Increasing secular trend of CLL incidence among all age groups for Taiwanese
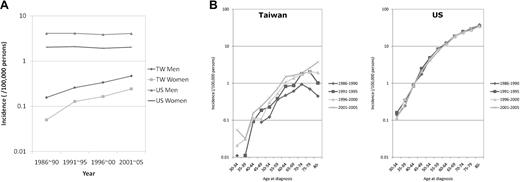
As shown in Figure 2A, for each age group of Caucasian Americans, the incidences remained rather stable during the study period; however, in Taiwanese, the incidence rates increased consistently over time in every age group. Comparison of the AAPC revealed that the estimated annual increase in incidences was statistically significantly positive in nearly all age groups for Taiwanese, indicating an increasing incidence trend of all ages (Figure 2B and supplemental Table; available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The quadratic term was not significant in all groups, implying that the trends of increasing incidence would be steady for Taiwanese (supplemental Table). On the contrary, the AAPC for Caucasian Americans was much smaller without a consistent pattern in every age group. No groups were statistically significant in either linear model or model with quadratic terms (Figure 2B and supplemental Table). These estimates were compatible with the increasing secular trends of incidences for Taiwanese and steady secular trends of incidences for Caucasian Americans (Figure 2A).
Secular trends. The secular (calendar) trends in age-specific rates (A) and comparison of the AAPC of incidence (B) of CLL in Taiwanese (TW) and Caucasian Americans (US) 1986 to 2005.
Secular trends. The secular (calendar) trends in age-specific rates (A) and comparison of the AAPC of incidence (B) of CLL in Taiwanese (TW) and Caucasian Americans (US) 1986 to 2005.
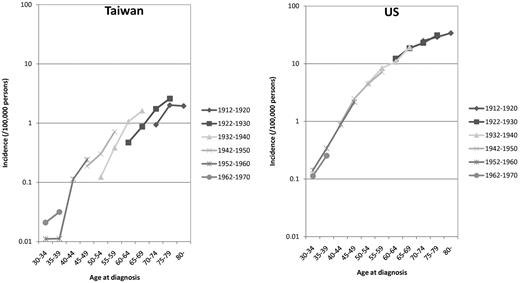
Higher CLL incidence in later birth cohorts for Taiwanese
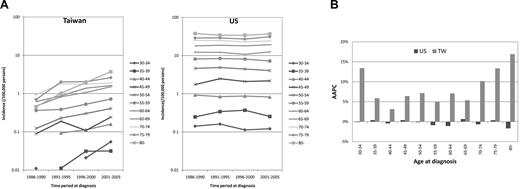
Figure 3 shows the age-specific incidences of CLL in representative birth cohorts. For Caucasian Americans, there were almost no differences in the incidences between earlier and later birth cohorts. For Taiwanese, in contrast, the incidence was higher for every later birth cohort in every given age group.
Age-specific incidence rates of representative birth cohorts in Taiwanese (TW) and Caucasian Americans (US) 1986 to 2005.
Age-specific incidence rates of representative birth cohorts in Taiwanese (TW) and Caucasian Americans (US) 1986 to 2005.
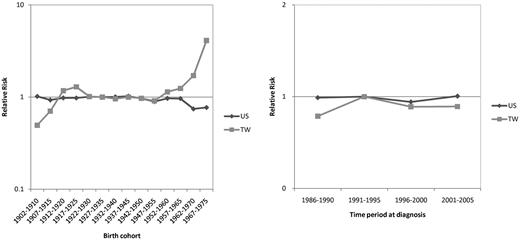
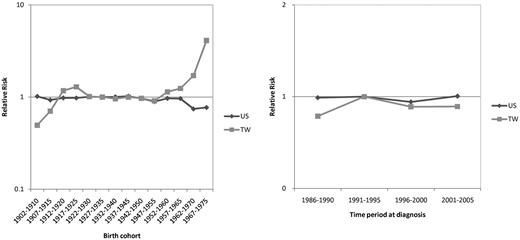
Significant birth-cohort effect in the CLL incidence trend for Taiwanese
The goodness-of-fit for different models are summarized in Table 1. For Taiwanese CLL data, the F tests showed statistically significant differences between the 2-factor (age-cohort or age-period) model and the age-alone model, indicating both a cohort effect and a period effect in the incidence trends. For Caucasian Americans, the difference between the age-period model and the age-alone model was significant; however, there was no significant difference between the age-cohort model and the age-alone model, suggesting that the period effect is stronger than the cohort effect. Figure 4 compares the relative risk of CLL between Taiwanese and Caucasian Americans in terms of cohort effect and period effect. Although data from model fitting suggested a period effect on the incidence trends in Caucasian Americans, the differences in relative risk between different time periods were not obvious; the curve of relative risk among various periods was virtually flat. A more prominent period effect, however, was seen in Taiwanese; the relative risk was highest during the period 1991-1995. The cohort effect was not seen in Caucasian Americans either; the curve of relative risk was nearly flat among different birth cohorts. In contrast, there was a prominent increase in relative risks in Taiwanese in the cohorts 1952-1960 onward, indicating the existence of a birth cohort effect.
Comparison of the birth cohort effects and period effects on the relative risk of CLL between Taiwanese (TW) and Caucasian Americans (US) 1986 to 2005.
Comparison of the birth cohort effects and period effects on the relative risk of CLL between Taiwanese (TW) and Caucasian Americans (US) 1986 to 2005.
Discussion
To our knowledge, this is the first report to compare the trends in incidences of CLL between Eastern and Western countries. The much lower incidences of CLL in Eastern countries, including Taiwan, are well known. But in this study, we have shown in addition that the incidence of CLL for Taiwanese has been increasing during the past 20 years, in contrast to the steady incidences in Caucasian Americans during the same period. Furthermore, the stronger birth-cohort effect in Taiwanese as shown by the APC model suggests that changes in lifestyle and environmental exposure between people born in earlier and later cohorts might play an important role in the development of CLL in this area.
In Taiwan, the major environmental changes in younger generations, compared with older generations, seemed to be the progressive westernization of lifestyle. Taiwan began to industrialize in the 1960s,13 which was also the time when the birth-cohort effect became more and more obvious (Figure 4). People born after 1960 might have been exposed to more high-calorie diets in their childhood, an event that resulted in an increased prevalence of overweight or obesity which has been reported to be associated with increased risk of CLL.15 Furthermore, industrialization might also have led to more exposure of chemical or physical hazards in these younger cohorts. Intriguingly, some chemical agents, such as pesticides, herbicides, hair dyes, or environmental tobacco and physical exposure, such as magnetic field radiation, have also been reported as associated with increased risk of CLL.5,16,,,,–21 These correlations provide the rational that the birth-cohort effect found in Taiwanese might be a consequence of environmental influences from the westernization of lifestyle. We also extracted the CLL incidence data from SEER for Asian/Pacific Islanders who are ethnically closer to Taiwanese but have been exposed to the westernized lifestyle. From 1991 to 2007 (supplemental Figure), the CLL incidence rate for male, female and both sexes among Asian/Pacific Islanders living in the United States was just in between that of Caucasian Americans and people living in Taiwan, suggesting the influence of both genetic background and lifestyle. This finding is also compatible with our notion that westernization of lifestyle may be associated with the cohort effect we have identified for Taiwanese.
The period effect on the trend of CLL incidence was also found stronger in Taiwanese by the APC model. Multiple factors might be involved in the period effect, including the improvement in diagnostic techniques and completeness of the cancer registry data. For Taiwanese, the estimation of the period effect showed that the risk was highest in the period 1991-1995 (Figure 4). Intriguingly, the extensive application of immunophenotyping assays of leukemic cells in clinical settings also began during this period in Taiwan. This improvement of diagnostic tools may be a contributing factor to the period effect.22,–24 Nonetheless, the diagnosis of CLL could be made by hemogram, cell morphology, and bone marrow examination for most patients with typical presentations such as marked leukocytosis and lymphocytosis before the era of immunophenotyping assays. The improved diagnostic techniques might only be helpful to find a few CLL patients with less degree of lymphocytosis. This may explain why the period effect is relatively minor in contrast to the strong birth cohort effect in Taiwan.
A major limitation of the analysis in this study is the difficulty in differentiating between a time-sustained period effect and a cohort effect. Because of the improvement in access to modern medical care, it is probable that the birth-cohort effect in Taiwanese is due, at least in part, to better disease detection. However, as shown in Figure 1B, the increased rate in younger patients was modest, compared with that of nearly 10-fold increase in older patients (> 80 years) during this period when all age groups had similarly better access to modern hospitals. In addition, access to medical care and improvement of diagnosis during that period were also supposed to be improving in the United States as well, but the incidence trends for Caucasian Americans remained steady. These findings argue against the improvement in disease detection as being a major factor in the increase in incidence of CLL in Taiwanese during the study period. Another limitation for this analysis is the completeness of individual registry systems. Two prior validation studies showed underreporting of CLL to the cancer registries.25,26 There has been no similar validation study for TNCR, but somewhat incompleteness of the registry data are expected. However, such underestimation of the true incidence rate might not influence the long-term incidence trend should the registry system be stable. The incidence curves of CLL for both Caucasian Americans and Taiwanese (Figure 1) were smooth without fluctuation, suggesting that the trends for both ethnic people, as well as the comparison between each other, were not significantly influenced by this ascertainment issue.
Albeit that there were differences of incidence trends, the sex-distribution pattern of CLL in Taiwanese was similar to that in Caucasian Americans (Figure 1A). Both populations have a male: female ratio of approximately 2:1. The increases in incidence in Taiwanese were parallel for males and females, suggesting that the contributing environmental factors had equal effects on both sexes. The most drastic increase in incidence was found in the older population of Taiwanese in which the peak incidence had shifted from 70-74 years to > 80 years, resulting in an age-specific incidence pattern similar to that of Caucasian Americans. This finding is also compatible with the notion that the incidence trends of CLL in Taiwanese might be caused by the westernization of lifestyle.
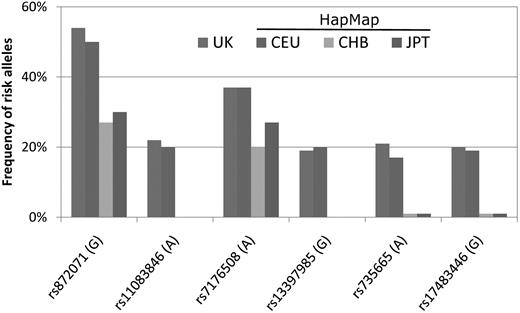
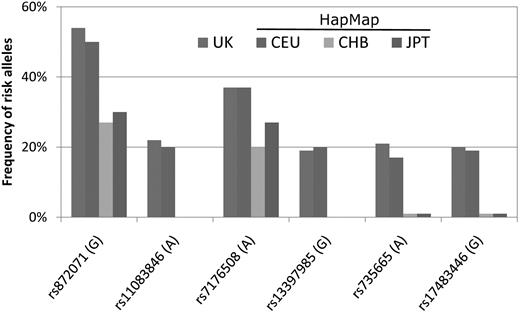
Although the incidence of CLL for Taiwanese has been increasing, it still remained much lower than that for Caucasian Americans. Genetic factors are believed to play a role in this racial disparity. Earlier studies demonstrated that the incidence of CLL remained as low for Asian immigrants in the United States as in their motherlands, suggesting the importance of racial genetic predisposition.5,27 Nonetheless, few studies have addressed this issue by direct comparison of the genetic profiles among Asians and Caucasians. In a recent genome-wide association study in the United Kingdom, Di Bernardo and Houlston and colleagues identified 6 susceptibility single nucleotide polymorphism loci for CLL.28 The association between these genetic susceptibility variants and CLL was validated in Caucasian Americans.29 It would be interesting if these genetic variants also have something to do with the lower risk of CLL development in Asian populations. The population frequencies of the risk alleles of these 6 loci in Asians can be obtained from the International HapMap Project database (http://snp.cshl.org/cgi-perl/gbrowse/hapmap27_B36). Interestingly, as shown in Figure 5, the risk alleles of 4 loci (rs17483466, rs13397985, rs735665, and rs11083846) were rarely seen, and those of the other 2 loci (rs872071 and rs7176508) were also less frequent in Han and Japanese people than in English people or Caucasians. This finding is compatible with the notion that racial differences in genetic background may partly explain the lower incidence of CLL in Asian countries, including Taiwan. Further studies, particularly in the field of molecular epidemiology, are warranted to uncover the genetic determinants of CLL.
The frequencies of risk alleles of 6 susceptibility single nucleotide polymorphism loci among different racial populations. The characters in parentheses indicate risk genotypes. The representative frequency in the United Kingdom was extracted from Di Bernardo et al28 ; the representative frequencies in Caucasians (CEU), Han people (CHB), and Japanese (JPT) were obtained from the International HapMap Project database. CEU indicates Utah residents with northern and western European ancestry from the Centre d'Etude du Polymorphisme Humain collection; CHB, Han Chinese in Beijing, China; and JPT, Japanese in Tokyo, Japan.
The frequencies of risk alleles of 6 susceptibility single nucleotide polymorphism loci among different racial populations. The characters in parentheses indicate risk genotypes. The representative frequency in the United Kingdom was extracted from Di Bernardo et al28 ; the representative frequencies in Caucasians (CEU), Han people (CHB), and Japanese (JPT) were obtained from the International HapMap Project database. CEU indicates Utah residents with northern and western European ancestry from the Centre d'Etude du Polymorphisme Humain collection; CHB, Han Chinese in Beijing, China; and JPT, Japanese in Tokyo, Japan.
From a broader point of view, the incidence of lymphomas, in particular B-cell lymphomas, is increasing worldwide.30,31 However, the trends of incidence are inconsistent among individual subtypes. For most subtypes of NHLs, there are increasing trends of incidence in both Western countries and Asian areas, including Taiwan.9,30,32 However, the incidence of myeloma is drastically increasing in Taiwan while it remains steady in the United States9,33,34 The incidence trends for Hodgkin lymphoma (HL) are not that consistent among Asian countries; in Japan, the incidence of HL was reported to be increasing,35 but no such trend was identified in Taiwan.9 These epidemiologic findings are in accordance with the fact that lymphoid malignancies are heterogeneous in nature with various etiologies; environmental factors may play roles in the carcinogenesis of some subtypes but not others. Furthermore, environmental factors and lifestyles are expected to take a long time, from years to decades, to influence the occurrence of a disease. This may explain why these factors show more influences on the incidence of myeloma and CLL, 2 diseases usually occurring in the elderly with a median age at diagnosis at approximately 65 to 70 years, than on that of other common lymphomas.
In conclusion, the incidence of CLL is much lower in Taiwanese than in Caucasian Americans, a finding that may be attributed to genetic disparity between races. Nonetheless, the incidence of CLL remains steady in Caucasian Americans but is drastically increasing in Taiwanese. Comparison of this trend distinction revealed that, in addition to age and calendar period, a birth-cohort effect is contributing to the increase of CLL incidence in Taiwan. This birth- cohort effect may reflect the consequence of lifestyle changes and may imply the existence of an environment influence on the leukemogenesis of CLL in Taiwan.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.-J.W., C.-J.C., and H.-F.T designed the research; S.-J.W., Y.-J.L., and C.-J.C. analyzed data; S.-Y.H. contributed vital material; and S.-J.W., C.-T.L., and H.-F.T wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chee-Jen Chang, Graduate Institute of Clinical Medical Science, Chang-Gung University, #5, Fu-Hsin St, Kwei-Shan, Tao-Yuan 333, Taiwan; e-mail: cjchang@mail.cgu.edu.tw; or Hwei-Fang Tien, Department of Internal Medicine, National Taiwan University Hospital, #7, Chung-Shan South Rd, Taipei 100, Taiwan; e-mail: hftien@ntu.edu.tw.