Abstract
Endothelial cells (ECs) represent a major source of actively secreted adenosine triphosphate (ATP). Natural killer (NK) cells can mediate vascular injury in several pathologic conditions, including cytomegalovirus infection and vascular leak syndrome. We studied NK-cell expression of P2 receptors and the role of these nucleotide receptors in the regulation of endothelial-NK cell cross-talk. NK cells from healthy subjects expressed P2Y1,2,4,6,11,12,13,14 and P2X1,4,5,6,7 receptors. NK cells stimulated with ATP, but not uridine triphosphate, increased intracellular Ca2+ and chemokinesis. Moreover, ATP, but not uridine triphosphate, inhibited NK chemotaxis in response to CX3CL1, whereas chemotaxis to CXCL12 was increased. CX3CL1 elicited killing of human umbilical vein ECs and human coronary artery ECs by NK cells. However, in the presence of ATP, CX3CL1 failed to stimulate killing of ECs. Such inhibitory effect was lost on exogenous addition of the ATP-hydrolyzing enzyme apyrase or by pharmacologic inhibition of the P2Y11R, and correlated with increased intracellular cyclic adenosine monophosphate concentrations induced by ATP or other P2Y11R agonists, including NAD+. Extracellular ATP regulates NK-cell cytotoxicity via P2Y11R activation, protecting ECs from CX3CL1-elicited NK cell–mediated killing. These findings point out the P2Y11R as a potential target for pharmacologic intervention aimed at reducing NK-mediated vascular injury.
Introduction
Nucleotides represent a class of molecules endowed with the ability to actively modulate specific cell processes.1 Nucleotides are released physiologically, as well as a consequence of cell damage, cell stress, bacterial, and parasite infection,2 and bind to specific plasma membrane receptors, named P2 receptors (P2R), widely distributed in eukaryotic cells. Intracellular pathways activated by P2Rs depend on the cell type, P2R subtypes expressed, and kind/amount of released nucleotide. Two P2 receptor subfamilies have been described so far and named P2YR and P2XR.3–5
P2XR are ligand-gated ion channels selective for monovalent and divalent cations. Seven different monomers have been cloned so far and named P2X1R to P2X7R. Differently from P2YR, the amino- and carboxyl-terminal domains of the P2XR subtypes are both cytoplasmic. On stimulation with adenosine triphosphate (ATP), P2XR subunits aggregate to form homomultimers or, in some cases, heteromultimers.5 They are expressed in mammalian sensory neurons, smooth muscle cells, fibroblasts, megakaryocytes, platelets, lymphocytes, macrophages, and dendritic cells.6 P2YR are 7 membrane-spanning, G-protein–coupled receptors whose activation triggers generation of inositol 1,4,5-trisphosphate and release of Ca2+ from the intracellular stores. Eight P2Y subtypes have been cloned so far (P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R).7 P2YR are expressed in platelets, mucosal cells, monocytes, macrophages, dendritic cells, neurons, smooth and striated muscle cells, etc. They are activated by different ligands depending on given subtype. P2Y1R, P2Y11R, P2Y12R, and P2Y13R are activated by ATP or adenosine diphosphate (ADP). P2Y2R is activated both by uridine triphosphate (UTP) and ATP; P2Y4R and P2Y6R have UTP and UDP as agonists, whereas UDP-glucose activates the P2Y14R subtype.
NK cells are bone marrow-derived lymphocytes that represent 5% to 10% of circulating peripheral blood mononuclear cells and contribute to the innate immune response by exerting cytolytic activity against virally infected and neoplastic cells and by secreting cytokines and chemokines. NK cells have been found in the advanced atherosclerotic lesions of human arteries as well as in the deep intima of diabetic patients.8 Moreover, NK cells are thought to mediate, at least in part, vascular damage occurring in vascular leak syndrome (VLS)9 and cytomegalovirus infection.10 In such conditions, CX3CL1 expressed by ECs recruits NK cells bearing CX3CR1 and stimulates excessive NK cell cytolytic activity leading to vascular damage.11 CX3CL1 is the only known member of the CX3C chemokine family containing the cysteine motif C-X-X-X-C. Expression of CX3CL1 on endothelial cells (ECs) is induced by inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interferon-γ, and plays a relevant role in leukocyte adhesion to the endothelium and transmigration in the inflamed tissue. Two isoforms of CX3CL1 have been described. As a membrane-anchored protein, CX3CL1 works as adhesion molecule strengthening leukocyte-EC interaction. As a secreted glycoprotein, CX3CL1 functions as a potent chemoattractant for CX3CR1-bearing cells, including T cells, monocytes, and NK cells. Moreover, CX3CR1 triggering by both soluble and membrane-bound isoforms, stimulates cytolytic activity of NK cells and CD8+ cytotoxic T cells on their way to the inflamed tissue.12
To date, the pattern of P2R expressed by NK cells has not been characterized. Here we show, for the first time, the expression profile of P2R by NK cells and the effect of extracellular nucleotides on NK-EC cross-talk.
Methods
Reagents and antibodies
ATP and UTP were obtained from Roche Molecular Biochemicals. Mouse anti–human fluorochrome-conjugated antibodies specific for CD56, CD16, CXCR4, and isotype control mouse immunoglobulin (Ig) were from BD Biosciences; monoclonal antibody (mAb) anti-CX3CR1 was obtained from MBL International. Recombinant human (rh) CXCL12, rhIL-2, and rhCX3CL1 were purchased from PeproTech. NF157 and NF023 were purchased from Tocris Bioscience and MRS2159 from Sigma-Aldrich.
Cells and cell culture
Peripheral blood mononuclear cells were isolated from healthy volunteers by Ficoll-Paque PLUS (GE Healthcare) density-gradient centrifugation. NK cells were isolated by negative immunomagnetic selection using Human NK Cell Enrichment cocktail (StemCell Technologies). NK-cell populations used in all experiments were more than 90% pure as confirmed by flow cytometric analysis. NK cells were maintained in RPMI 1640 growth medium containing 10% of fetal bovine serum, 1% l-glutamine, 50 IU/mL penicillin, and 50 μg/mL streptomycin (complete medium). The cell line K562 was obtained from ATCC. Human umbilical vein ECs (HUVECs) and human coronary artery ECs (HCAECs) were obtained from Lonza.
Reverse-transcriptase polymerase chain reaction
Total RNA was isolated from NK cells by TRIzol (Invitrogen). A total of 1 μg of RNA was retro-transcribed using random primers and incubated with 200 U of M-MLV-RT. cDNAs were then subjected to polymerase chain reaction. Primer pairs sequences for P2XR and P2YR subtypes and polymerase chain reaction conditions were previously described.13
Cytosolic Ca2+ concentration measurements
Cells (1 × 107/mL) were loaded with fura-2/AM (4μM) for 15 minutes at 37°C in a saline solution containing: 125mM NaCl, 5mM KCl, 1mM MgSO4, 1mM Na2HPO4, 5.5mM glucose, 5mM NaHCO3, 1mM CaCl2, and 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.4 with NaOH). Cells were rinsed and incubated in a Na+-free saline solution (300mM sucrose, 1mM K2HPO4, 1mM MgSO4, 5.5mM glucose, 1mM CaCl2, and 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, with KOH) in a temperature-controlled magnetically stirred cuvette at 37°C. [Ca2+]i changes were measured with a PerkinElmer fluorometer (PerkinElmer Inc). The excitation wavelength was at 340 to 380 nm, and emission at 510 nm. Ca2+ concentration was calculated using the FLwinlab software (PerkinElmer Inc).14
Flow cytometric analysis of NK cells
Cells were incubated with fluorochrome-conjugated Abs for 20 minutes at 4°C, washed and resuspended in fluorescence-activated cell sorter buffer. Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences). Results are shown as net arithmetical mean fluorescence intensity, calculated by subtracting the mean fluorescence obtained with isotype-matched control Ig from the mean fluorescence measured by mAbs.
Migration assays
Chemotaxis was evaluated by measuring migration through 5-μm pore polycarbonate filters in 24-well transwell chambers. Different concentrations of chemokines and/or nucleotides were added in the bottom chamber, and 2 × 105 NK cells were resuspended in 0.5% bovine serum albumin containing RPMI 1640 in the top chamber. Cells were allowed to migrate into the bottom chamber for 60 minutes at 37°C, harvested and counted using a FACSCalibur device for 90 seconds at a flow rate of 60 μL/min. Results are shown as migration index, representing the ratio between cells migrated in the presence of indicated stimuli and cells migrated in response to medium alone.
Cytotoxicity assay
K562 cells were incubated with 120 μCi of Na2(51Cr)O4 (GE Healthcare), washed, and cocultured at 5 × 103 cells/well in 96-well microtiter plates with NK cells in triplicate at the indicated effector/target ratios. After 4-hour incubation at 37°C, supernatants were harvested, and radioactivity measured using a γ-counter.
In NK-EC cytotoxicity assays, HUVECs or HCAECs were resuspended at 1 × 106 cells/mL. In some experiments, cell suspension was depleted of dead cells by immunomagnetic beads (Miltenyi Biotec). ECs were then incubated with 15μM calcein-AM (Invitrogen) for 30 minutes at 37°C. After 2 washes, cells were seeded at 5 × 103 cells/well in 96-well microtiter plates with NK cells in triplicate at the indicated effector/target ratios. After 4-hour incubation, 75 μL of supernatant was transferred into 96-well optical plate and fluorescence measured using a Tecan Infinite fluorometer. Maximal and spontaneous release from target cells was obtained plating target cells in 0.2 mL of complete medium with or without 1% Triton X-100, respectively. Specific cell lysis was calculated as follows: lysis (%) = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Spontaneous release was in each experiment less than 8% of the maximum. NK cells were stimulated with UTP, ATP, or ATP analogs for 1 hour and extensively washed before coculture with target cells.
Intracellular cAMP quantification
Cyclic adenosine monophosphate (cAMP) assays were performed using a cAMP direct immunoassay kit (BioVision) following the manufacturer's instructions. Briefly, freshly extracted NK cells (8 × 106/well) were left untreated or incubated with 100μM ATP for 15 minutes with or without 30 minutes of pretreatment with 100μM NF175, 10μM MRS2159, or 1μM NF023. Cells were harvested washed, lysed, and centrifuged. Supernatants and standards provided with the kit assessed in triplicate in a protein G–coated assay plate and incubated with anti-cAMP pAb for 1 hour at room temperature. cAMP-horseradish peroxidase was then added to samples and incubated for 1 hour at room temperature; wells were extensively washed before the addition of horseradish peroxidase developer solution and further incubation for 1 hour. Reactions were stopped adding 1N HCl and optical densities read immediately at 450 nm.
Results
NK cells express members of the P2X and P2Y receptor subfamilies
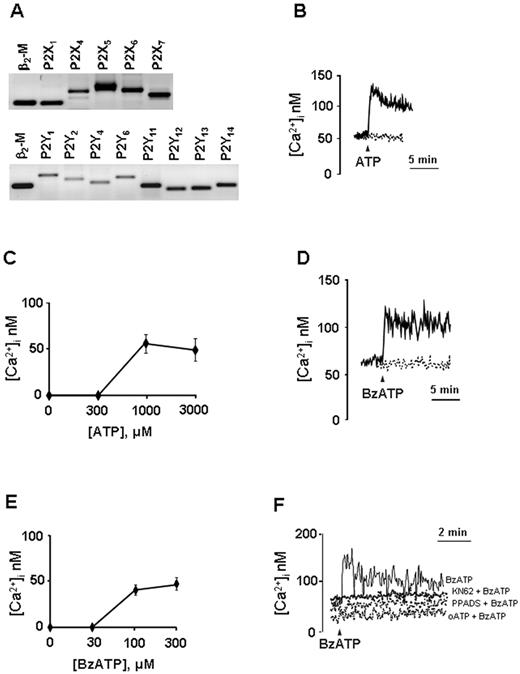
Freshly isolated NK cells from healthy donors were studied for the expression of mRNA encoding for P2R. Discrete amounts of mRNA were detected for P2X1R, P2X4R, P2X5R, P2X6R, P2X7R and P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, P2Y14R subtypes as shown in Figure 1A. On the contrary, P2X2R and P2X3R mRNAs were not expressed in NK cells from any of the donors studied (data not shown; and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
NK cells express functional P2Rs. (A) Expression profile of mRNA encoding P2XR and P2YR and the housekeeping gene β2 microglobulin (β2-M) in freshly isolated NK cells, 1 representative experiment of 4 is shown. (B,D) Calcium signal in NK cells stimulated with extracellular nucleotides. Cells were stimulated with 1mM ATP (B) or 300μM BzATP (D) in the presence (continuous line) or absence (dashed line) of extracellular Ca2+. For Ca2+-free conditions, 0.5mM ethyleneglycoltetraacetic acid was added to the Ca2+-free saline solution to chelate contaminating Ca2+. Dose-dependency curves for ATP- (C) or BzATP-induced (E) Ca2+ response are shown. (F) NK cells were incubated in the presence of the following P2 antagonists/inhibitors: KN62 (100nM, for 30 minutes), PPADS (100μM, for 2 hours), and oATP (600μM for 1.5 hours). Cells were rinsed (oATP and PPADS) and stimulated with 300μM BzATP. One representative experiment of at least 5 similar is shown (B,D,F). Data are mean ± SD of at least 4 independent experiments (C,E).
NK cells express functional P2Rs. (A) Expression profile of mRNA encoding P2XR and P2YR and the housekeeping gene β2 microglobulin (β2-M) in freshly isolated NK cells, 1 representative experiment of 4 is shown. (B,D) Calcium signal in NK cells stimulated with extracellular nucleotides. Cells were stimulated with 1mM ATP (B) or 300μM BzATP (D) in the presence (continuous line) or absence (dashed line) of extracellular Ca2+. For Ca2+-free conditions, 0.5mM ethyleneglycoltetraacetic acid was added to the Ca2+-free saline solution to chelate contaminating Ca2+. Dose-dependency curves for ATP- (C) or BzATP-induced (E) Ca2+ response are shown. (F) NK cells were incubated in the presence of the following P2 antagonists/inhibitors: KN62 (100nM, for 30 minutes), PPADS (100μM, for 2 hours), and oATP (600μM for 1.5 hours). Cells were rinsed (oATP and PPADS) and stimulated with 300μM BzATP. One representative experiment of at least 5 similar is shown (B,D,F). Data are mean ± SD of at least 4 independent experiments (C,E).
Ca2+ responses induced by P2Rs expressed by NK cells
Expression of P2R by NK cells was confirmed by pharmacologic analysis. Binding of extracellular nucleotides to P2R elicits an increase in the intracellular Ca2+ concentration ([Ca2+]i) that is known to cause cellular actin polymerization, which is causally linked to cell motility (chemotaxis, chemokinesis). We therefore studied Ca2+ signaling in nucleotide-stimulated NK lymphocytes. Cells were loaded with the fluorescent Ca2+ indicator fura-2/AM, and intracellular Ca2+ changes were monitored by fluorometric analysis. Stimulation of NK cells with nucleotides in a standard (ie, Na+-containing, “Cystolic Ca2+ concentration measurements”) saline solution did not induce an appreciable Ca2+ signal (not shown). It is known that Na+ and Ca2+ compete for the entry through P2XR channels.15 To avoid competition between the 2 ions thus amplifying Ca2+ responses, [Ca2+]i changes were measured in cells incubated in a Na+-free saline solution (ie, where Na+ was replaced by sucrose, “Cystolic Ca2+ concentration measurements”). Figure 1B shows that ATP triggered in NK cells a spiking [Ca2+]i increase followed by a sustained plateau (ΔCa2+ = 54.2nM ± 12.8); although in the absence of the extracellular cation, Ca2+ peak and plateau were absent (dashed line), indicating that the great (if not all) Ca2+ ions contributing to the ATP-induced [Ca2+]i increase derived from influx through the P2XR channels and not from the intracellular Ca2+ stores (P2YR-mediated response). Figure 1C shows that ATP threshold concentration for the Ca2+ response was quite high (ie, ∼ 300μM), whereas the maximal response was obtained with 1mM ATP. Interestingly, α,β-meATP, a potent agonist of the P2X1R, did not induce any [Ca2+]i increase (not shown), suggesting that, although NK cells expressed detectable P2X1R mRNA, no functional receptor was present on cell membrane.
We also tested the ATP analog BzATP, an agonist that preferentially, although not exclusively, activates the P2X7R subtype. Figure 1D (solid line) shows that, similarly to ATP, 300μM BzATP induced a rapid [Ca2+]i increase that remained sustained for several minutes. Because it is reported that BzATP is also active at the P2Y11R subtype, we also performed stimulation of NK cells with this agonist in Ca2+-free conditions. Figure 1D (dashed line) shows that, when BzATP was applied in the absence of extracellular Ca2+, no peak was detected. The BzATP dose-dependency curve is shown in Figure 1E. BzATP threshold concentration was approximately 30μM, whereas ΔCa2+ at 300μM BzATP was 48.1 plus or minus 5.0nM. Different P2XR inhibitors are available; among them, oxidized ATP (oATP) and pyridoxalphosphate-6-azophenyl 2-disulfonic acid (PPADS) are recognized as P2XR wide spectrum inhibitors, whereas KN-62 is a P2X7R noncovalent blocker. oATP, PPADS, and KN-62 completely abrogated the [Ca2+]i increase induced by BzATP in NK cells (Figure 1F).
As P2YR can be activated by different agonists depending on the subtype, we checked the response induced by 2MeSADP (an agonist at P2Y1R), which induced no Ca2+ increase or, in some subjects, a delayed/slowly increasing Ca2+ signal (not shown). ADP that activates P2Y1R, P2Y12R, and P2Y13R and UTP, which is an agonist at the P2Y2R, P2Y4R, and P2Y6R subtypes, did not induce any appreciable Ca2+ increase, as well as UDP, which is very active at the P2Y6R subtype and UDP-glucose (agonist at P2Y14R; not shown). The nonhydrolyzable ATP analog ATP-γS, which is an agonist at the P2Y11R subtype, gave an appreciable Ca2+ signal only in some of the subjects tested (not shown). However, as the P2Y11R has the unique (among P2Rs) ability to induce increased intracellular cAMP (cAMPi) concentration in addition to mobilizing intracellular calcium, the lack of intracellular Ca2+ mobilization on ATP-γS stimulation is not sufficient to exclude P2Y11R activation.
ATP, but not UTP, influences NK cell motility and chemotaxis
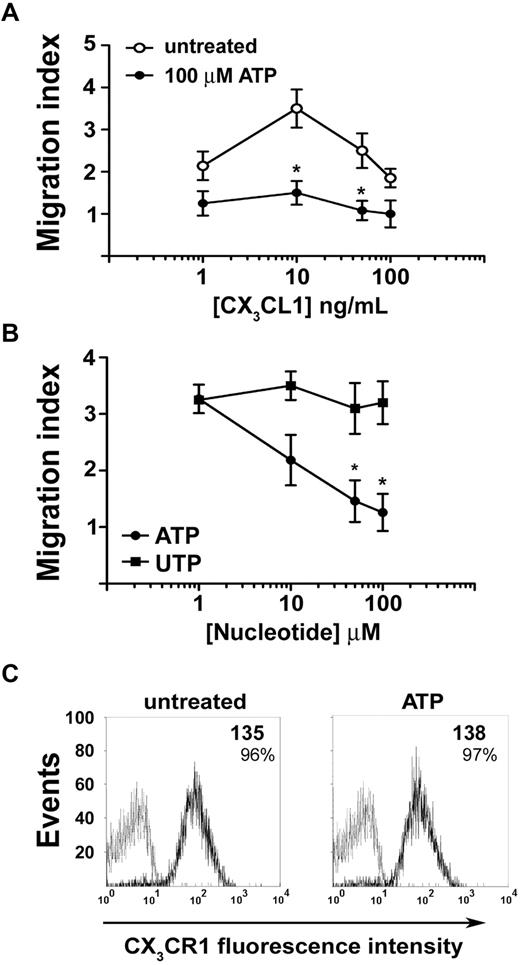
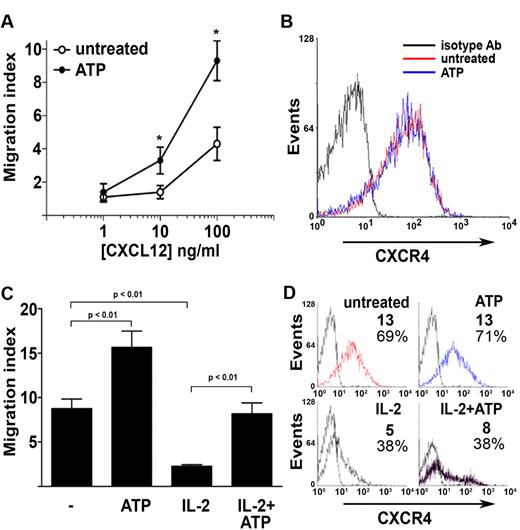
We asked whether extracellular nucleotides could affect migratory properties of NK cells. Neither ATP nor UTP was able to induce chemotaxis of NK cells in a concentration range spanning from 1nM to 100μM (data not shown), yet ATP (but not UTP) increased NK-cell nondirectional motility (chemokinesis) as shown by checkerboard analysis (Table 1). However, exposure to extracellular ATP (but not UTP) markedly modified NK-cell migratory response to chemokines. Ninety percent of circulating NK cells were CD16+ and expressed CX3CR1 and CXCR4 chemokine receptors. In vitro chemotaxis assays showed dose-dependent NK-cell migration induced by CX3CL1. However, in the presence of 100μM ATP, CX3CL1-induced chemotaxis was completely blocked (Figure 2A). Moreover, ATP-induced block of chemotaxis was dose-dependent, whereas UTP failed to alter NK-cell migration (Figure 2B). Flow cytometric analysis showed that ATP did not significantly alter CX3CR1 expression on NK-cell membrane (Figure 2C). CX3CR1 mean fluorescence intensity remained relatively high, and the percentage of CX3CR1+ NK cells was unchanged and proximal to 100%, thus indicating that ATP did not inhibit CX3CL1-mediated effects through CX3CR1 down-regulation. In contrast with the inhibition exerted by ATP on CX3CL1-induced chemotaxis, NK cells migrated more vigorously to CXCL12 in the presence of ATP rather than in its absence (Figure 3A). This effect was not the result of acute CXCR4 up-regulation as shown in Figure 3B. Similarly, chronic (16 hours) stimulation with ATP enhanced responsiveness to CXCL12 of resting and IL-2–activated NK cells (Figure 3C). In such stimulation conditions, ATP alone did not alter CXCR4 expression (Figure 3D top panels); however, IL-2–induced receptor down-regulation was partially prevented by the presence of ATP (Figure 3D bottom panels), and this effect correlated to chemotactic response to CXCL12. Taken together, these data show that extracellular ATP exerts distinct regulatory effects on NK-cell chemotaxis in response to CX3CL1 or CXCL12. Moreover, ATP-dependent enhancement of chemokinesis and chemotaxis to CXCL12 indicates that ATP-induced reduction of NK-cell response to CX3CL1 is not the result of a toxic effect. Intrinsic ATP toxicity was further excluded by flow cytometric analysis of NK cells stained with annexin V and propidium iodide for the detection of early apoptotic and late apoptotic or necrotic cells (supplemental Figure 2). Cell death was not detected at the ATP concentration used to stimulate NK cells (100μM) but only occurred at concentrations as high as 1mM.
ATP inhibits chemotaxis of NK cells induced by CX3CL1. (A) Migratory response of NK cells to CX3CL1 gradients in the presence (●) or absence (○) of 100μM ATP. *P < .05 versus cells stimulated with the same dose of CX3CL1 in the absence of ATP as assessed by Student t test for paired samples. (B) Dose dependency of the inhibitory effect of ATP or UTP on the migration of NK cells to CX3CL1. NK lymphocytes migrated to 10 ng/mL CX3CL1 added to the bottom chamber along with the indicated concentrations of ATP (●) or UTP (■). *P < .05 versus cells stimulated with UTP assessed by Student t test for paired samples. (C) Flow cytometric analysis of the expression of CX3CR1 on the membrane of NK cells left untreated or exposed to 100μM ATP for 60 minutes. Numbers represent the net mean fluorescence calculated subtracting the mean fluorescence obtained with isotype control mAb (gray line) to the fluorescence obtained with anti-CX3CR1 mAb (black line). (A-B) Data are mean ± SD from 4 experiments. In all chemotaxis assays, each concentration used of CX3CL1 induced statistically significant increase of NK-cell chemotaxis compared with the unstimulated cells. P < .05 assessed by Student t test for unpaired samples. (C) Representative results from 1 of 3 experiments.
ATP inhibits chemotaxis of NK cells induced by CX3CL1. (A) Migratory response of NK cells to CX3CL1 gradients in the presence (●) or absence (○) of 100μM ATP. *P < .05 versus cells stimulated with the same dose of CX3CL1 in the absence of ATP as assessed by Student t test for paired samples. (B) Dose dependency of the inhibitory effect of ATP or UTP on the migration of NK cells to CX3CL1. NK lymphocytes migrated to 10 ng/mL CX3CL1 added to the bottom chamber along with the indicated concentrations of ATP (●) or UTP (■). *P < .05 versus cells stimulated with UTP assessed by Student t test for paired samples. (C) Flow cytometric analysis of the expression of CX3CR1 on the membrane of NK cells left untreated or exposed to 100μM ATP for 60 minutes. Numbers represent the net mean fluorescence calculated subtracting the mean fluorescence obtained with isotype control mAb (gray line) to the fluorescence obtained with anti-CX3CR1 mAb (black line). (A-B) Data are mean ± SD from 4 experiments. In all chemotaxis assays, each concentration used of CX3CL1 induced statistically significant increase of NK-cell chemotaxis compared with the unstimulated cells. P < .05 assessed by Student t test for unpaired samples. (C) Representative results from 1 of 3 experiments.
ATP enhances NK-cell chemotactic response to CXCL12. (A) Migratory response of NK cells to CXCL12 gradients in the presence (●) or absence (○) of 100μM ATP. *P < .05 versus cells stimulated with same CXCL12 dose in the presence of UTP. (B) Expression of CXCR4 on NK cells surface after 1-hour exposure to 100μM ATP. (C) Migratory response of NK cells to 100 ng/mL CXCL12 after 16-hour incubation with or without 1000 U/mL IL-2 in the presence or absence of 100μM ATP. (D) Expression of CXCR4 after 16-hour incubation with or without 1000 U/mL IL-2 in the presence or absence of 100μM ATP. (D) Data are the mean fluorescence intensity and percentages of CXCR4+ cells. (A,C) Data are the mean ± SD of 3 experiments. (B,D) Data are representative from 1 of 3 experiments. *P < .05. All P values were calculated by Student t test for paired samples.
ATP enhances NK-cell chemotactic response to CXCL12. (A) Migratory response of NK cells to CXCL12 gradients in the presence (●) or absence (○) of 100μM ATP. *P < .05 versus cells stimulated with same CXCL12 dose in the presence of UTP. (B) Expression of CXCR4 on NK cells surface after 1-hour exposure to 100μM ATP. (C) Migratory response of NK cells to 100 ng/mL CXCL12 after 16-hour incubation with or without 1000 U/mL IL-2 in the presence or absence of 100μM ATP. (D) Expression of CXCR4 after 16-hour incubation with or without 1000 U/mL IL-2 in the presence or absence of 100μM ATP. (D) Data are the mean fluorescence intensity and percentages of CXCR4+ cells. (A,C) Data are the mean ± SD of 3 experiments. (B,D) Data are representative from 1 of 3 experiments. *P < .05. All P values were calculated by Student t test for paired samples.
Extracellular ATP blocks the ability of CX3CL1 to stimulate NK-cell cytotoxicity
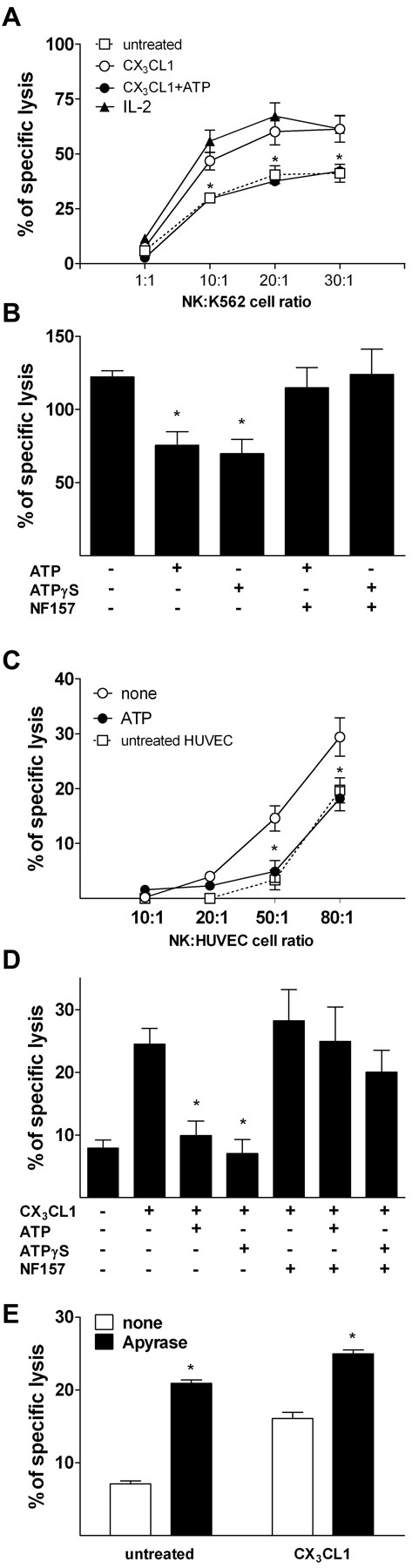
As ATP inhibited CX3CL1-induced NK-cell migration, we sought to determine whether ATP also affected CX3CL1 stimulation of NK-cell cytotoxicity. First, we evaluated the effect of the exposure to ATP on NK cell–mediated cytolysis of the monocytic cell line K562, a classic cellular target for in vitro NK cytotoxicity studies. In keeping with previous observations, soluble CX3CL1 increased NK cell–mediated killing of K562 target cells9 (Figure 4A). However, in the presence of 100μM ATP, CX3CL1 failed to enhance NK-cell cytolytic activity. Similarly to what was observed for chemotaxis, CX3CL1 concentrations as low as 1 ng/mL significantly enhanced NK-cell cytotoxicity; however, maximal stimulation was obtained using 10 ng/mL CX3CL1, and we therefore used such dose hereinafter in all experiments.
ATP inhibits CX3CL1-induced NK-cell cytotoxicity. (A) Effect of 100μM ATP on 10 ng/mL CX3CL1-induced NK cell–mediated killing of K562 cells *P < .05, CX3CL1 versus CX3CL1 + ATP. (B) CX3CL1 ability to increase HUVEC killing by NK cells is blocked by ATP and ATP-γS and restored by NF157. NK cells were treated with 10 ng/mL CX3CL1 in the presence of the indicated stimuli (100μM ATP, 10μM ATP-γS, 100μM NF157) for 2 hours before the coculture performed at 80:1 (NK/target cells) ratio. *P < .05 versus cells treated with CX3CL1 only. (C) ATP (100μM) inhibits NK cell–mediated cytolytic activity against TNF-α–activated ECs. HUVECs were left untreated or stimulated for 16 hours with 50 ng/mL rhTNF-α and used in cytotoxicity assay. *P < .05 versus cells treated with TNF-α only. (D) Effect of 100μM ATP and 10μM ATP-γS on CX3CL1-induced killing of HCAECs by NK cells. *P < .05 versus cells treated with CX3CL1 only. (E) Hydrolysis of endogenous extracellular ATP by apyrase (1 U/mL) enhances basal and CX3CL1-induced NK cell–mediated killing of HUVECs. *P < .05. Data are mean ± SD of percentages of unstimulated NK cells from 3 independent experiments. All P values were calculated by Student t test for paired samples.
ATP inhibits CX3CL1-induced NK-cell cytotoxicity. (A) Effect of 100μM ATP on 10 ng/mL CX3CL1-induced NK cell–mediated killing of K562 cells *P < .05, CX3CL1 versus CX3CL1 + ATP. (B) CX3CL1 ability to increase HUVEC killing by NK cells is blocked by ATP and ATP-γS and restored by NF157. NK cells were treated with 10 ng/mL CX3CL1 in the presence of the indicated stimuli (100μM ATP, 10μM ATP-γS, 100μM NF157) for 2 hours before the coculture performed at 80:1 (NK/target cells) ratio. *P < .05 versus cells treated with CX3CL1 only. (C) ATP (100μM) inhibits NK cell–mediated cytolytic activity against TNF-α–activated ECs. HUVECs were left untreated or stimulated for 16 hours with 50 ng/mL rhTNF-α and used in cytotoxicity assay. *P < .05 versus cells treated with TNF-α only. (D) Effect of 100μM ATP and 10μM ATP-γS on CX3CL1-induced killing of HCAECs by NK cells. *P < .05 versus cells treated with CX3CL1 only. (E) Hydrolysis of endogenous extracellular ATP by apyrase (1 U/mL) enhances basal and CX3CL1-induced NK cell–mediated killing of HUVECs. *P < .05. Data are mean ± SD of percentages of unstimulated NK cells from 3 independent experiments. All P values were calculated by Student t test for paired samples.
Next, we asked whether the block of CX3CL1-dependent increase of NK-cell cytotoxicity was reproducible using ECs as target cells (Figure 4B). ATP alone did not significantly alter basal NK-cell killing activity (not shown). However, in the presence of ATP, or of the nonhydrolyzable ATP analog ATP-γS, CX3CL1 failed to stimulate killing of HUVECs. Pretreatment with NF157, a compound that selectively blocks the P2Y11R and the P2X1R, restored CX3CL1 ability to stimulate cytolysis of HUVECs in NK cells exposed to ATP or ATP-γS. This finding suggests that ATP-dependent block of CX3CL1 stimulatory effects was mediated by P2X1R and/or P2Y11R.
Stimulation of HUVECs with TNF-α enhances surface expression of CX3CL1 as well as expression of enzymes mediating CX3CL1 cleavage and shedding.16–18 Therefore, we compared the susceptibility of TNF-α–activated HUVECs with NK-cell cytolysis in the presence or absence of ATP. HUVECs are relatively resistant to NK cell–mediated cytolysis, and significant cytolytic activity is measurable at higher effector/target ratios compared with those needed for K562 cells. Activated HUVECs displayed increased susceptibility to NK cell–dependent cytolysis that was already detectable at an effector/target ratio as low as 20:1. Lysis of untreated HUVECs was undetectable at this level. However, cytotoxicity toward TNF-α–activated HUVECs by NK cells pre-exposed to ATP was superimposable with that of NK cells toward unstimulated HUVECs (Figure 4C).
We also performed cytotoxicity assays using HCAECs, a second EC line as a target for NK cell–mediated cytotoxicity. ATP and ATP-γS also inhibited the ability of CX3CL1 to stimulate NK-cell killing of HCAECs, confirming the results obtained with HUVECs (Figure 4D). Moreover, the killing of HCAECs by NK cells exposed to ATP or ATP-γS was restored by NF157, again indicating the involvement of the P2Y11R and/or P2X1R as mediators of ATP-dependent NK-cell function inhibition.
As ECs can secrete discrete amounts of ATP in the extracellular space, we sought to determine whether such secretion was able to affect NK cell–mediated killing of ECs. Therefore, we performed cytotoxicity assays coculturing NK cells and HUVECs in the presence or absence of the ATP-hydrolyzing enzyme apyrase. The addition of apyrase to HUVEC culture 1 hour before or at time of the addition of NK cells dramatically enhanced the lysis of ECs (Figure 4E).
Inhibition of NK-cell response to CX3CL1 is mediated by the P2Y11 receptor
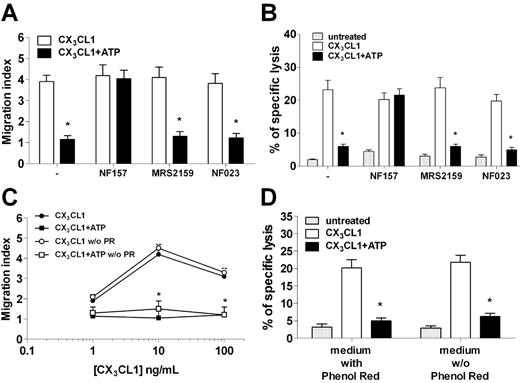
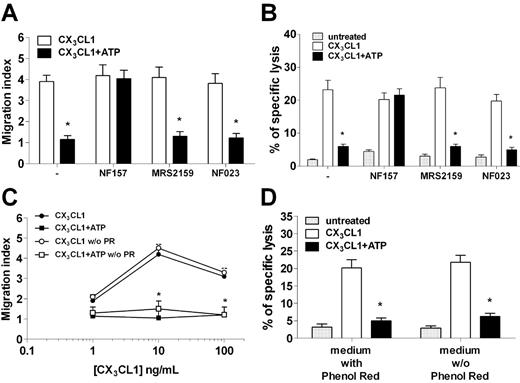
As NF157 can inhibit both P2Y11R and P2X1R, we next conducted a series of experiments to assess whether both receptors are involved in mediating ATP-dependent inhibitory effects. We then studied ATP inhibition of NK chemotaxis to CX3CL1 in the presence of NF157, or MRS2159 and NF023, 2 different P2X1 inhibitors.19–22 Although NF157 restored NK chemotaxis abolished by ATP, neither MRS2159 nor NF023 modified NK-cell migration to CX3CL1 in the presence of ATP (Figure 5A). Similarly, NK-cell killing of HUVECs that was elicited by CX3CL1 and blocked by ATP was restored by NF157 but not by P2X1 antagonists (Figure 5B). In addition, the medium used to culture NK cells (RPMI 1640) contained 13.3μM phenol red, a concentration 4.3-fold higher than the reported 50% inhibitory concentration for P2X1.23 Therefore, we assessed whether phenol red might have modified ATP effect on NK cells repeating chemotaxis and cytotoxicity assays in a phenol red–free medium. Results shown in Figure 5C-D indicate that phenol red did not affect ATP capacity to block migration or cytotoxicity of NK cells in response to CX3CL1. In keeping with the lack of functional P2X1R, α,β-meATP, an agonist at the P2X1R but not at the P2Y11R, did not modify chemotaxis or cytotoxicity of NK cells in response to CX3CL1 (data not shown). Taken together, these results suggested that P2Y11R, but not P2X1R, mediated the inhibition of NK-cell responsiveness to CX3CL1 exerted by ATP.
NF157, but not P2X1R, specific antagonists MRS2159 and NF023 restore NK responsiveness to CX3CL1. (A) NK-cell chemotaxis to 10 ng/mL CX3CL1 in the presence or absence of 100μM ATP with or without 30-minute pretreatment with 100μM NF157, 10μM MRS2159, or 1μM NF023. *P < .05, CX3CL1 versus CX3CL1 + ATP. (B) NK-cell killing of HUVECs after stimulation with or without 10 ng/mL CX3CL1 in the presence or absence of ATP and the indicated inhibitors. *P < .05, CX3CL1 versus CX3CL1 + ATP. (C) Chemotaxis of NK cells to CX3CL1 with or without ATP and in the presence or absence of phenol red. *P < .05, CX3CL1 versus CX3CL1 + ATP. (D) NK-cell killing of HUVECs stimulated or not with CX3CL1 with or without ATP and the presence or absence of phenol red. *P < .05, CX3CL1 versus CX3CL1 + ATP.
NF157, but not P2X1R, specific antagonists MRS2159 and NF023 restore NK responsiveness to CX3CL1. (A) NK-cell chemotaxis to 10 ng/mL CX3CL1 in the presence or absence of 100μM ATP with or without 30-minute pretreatment with 100μM NF157, 10μM MRS2159, or 1μM NF023. *P < .05, CX3CL1 versus CX3CL1 + ATP. (B) NK-cell killing of HUVECs after stimulation with or without 10 ng/mL CX3CL1 in the presence or absence of ATP and the indicated inhibitors. *P < .05, CX3CL1 versus CX3CL1 + ATP. (C) Chemotaxis of NK cells to CX3CL1 with or without ATP and in the presence or absence of phenol red. *P < .05, CX3CL1 versus CX3CL1 + ATP. (D) NK-cell killing of HUVECs stimulated or not with CX3CL1 with or without ATP and the presence or absence of phenol red. *P < .05, CX3CL1 versus CX3CL1 + ATP.
ATP inhibits NK-cell responsiveness to CX3CL1 via increased amount of intracellular cAMP
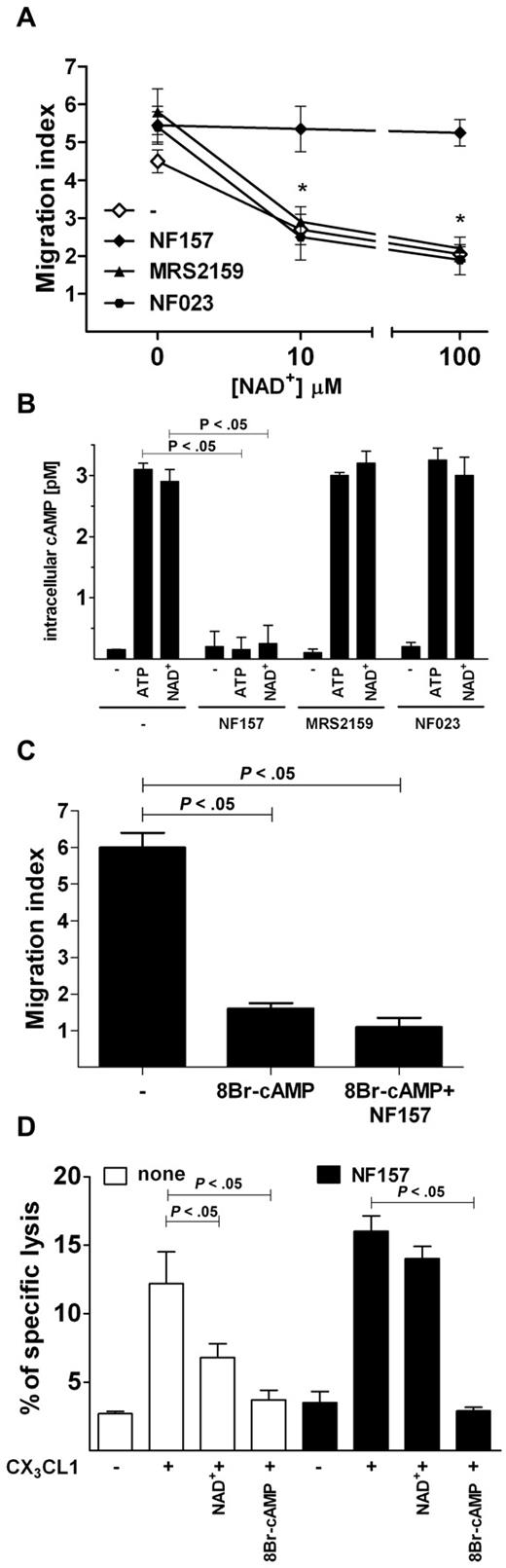
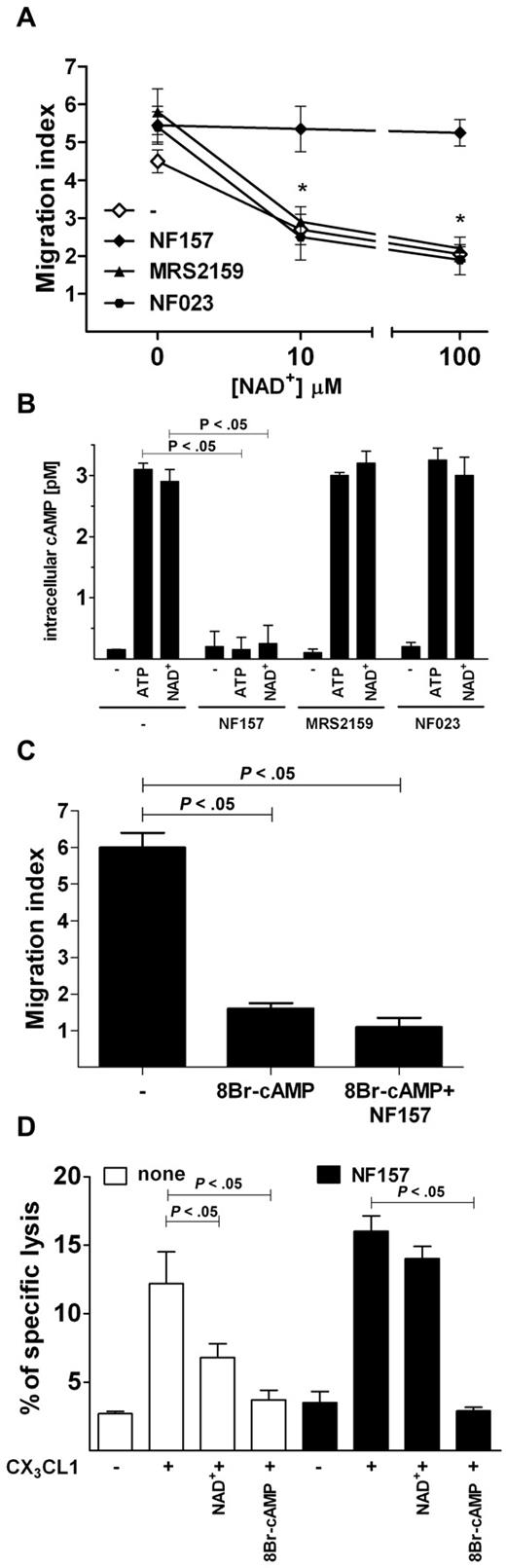
In the attempt to identify the receptor mediating the inhibitory effects of ATP, we assessed whether NAD+, another physiologic agonist at the P2Y11 receptor,24 was able to mimic ATP in blocking NK chemotaxis toward CX3CL1. NAD+ dose-dependently inhibited NK-cell chemotaxis to CX3CL1; however, when NK cells were preincubated with NF157 but not with MRS2159 or NF023, NAD+ was unable to block NK-cell migration (Figure 6A). Because the P2Y11R is the only P2R linked to adenylate cyclases activation, we assessed whether ATP or NAD+ was able to increase intracellular cAMPi concentration in NK cells. After a 15-minute incubation with either ATP or NAD+, NK cells displayed dramatically increased cAMPi levels. Such an effect was abolished by preincubation with NF157 but not MRS2159 or NF023 (Figure 6B). We next asked whether NK chemotaxis to CX3CL1 was inhibited by increased cAMPi concentration. Therefore, cells were incubated with the cell-permeable cAMP analog 8Br-cAMP before the stimulation with CX3CL1. Increasing doses of 8Br-cAMP inhibited NK-cell chemotaxis to CX3CL1, and coincubation with NF157 was unable to restore cell migration (Figure 6C). Similarly, NF157 restored CX3CL1-stimulated killing of HUVEC cell line by NK cells exposed to NAD+ but not to 8Br-cAMP (Figure 6D), indicating that P2Y11R activation negatively regulates CX3CR1 signaling, possibly by increasing cAMPi.
P2Y11R activation increases cAMPi and mediates ATP-induced inhibition of CX3CL1-elicited chemotaxis and cytotoxicity of NK cells. (A) Effect of NAD+ on CX3CL1-induced NK-cell chemotaxis. Cells were preincubated with or without the indicated inhibitors, stimulated with 10 ng/mL CX3CL1 alone in the presence of the indicated concentrations of NAD+ for 60 minutes and cell migration measured as indicated in “Migration assays.” *P < .05, MRS2159 or NF023 versus NF157. (B) Induction of cAMPi by ATP and NAD+. NK cells were incubated at 37°C for 15 minutes with 100μM ATP or NAD+ in the presence or absence of the indicated inhibitors, and concentration of cAMPi was measured as described in “Intracellular cAMP quantification.” (C) Inhibition of CX3CL1-dependent NK-cell migration by 8Br-cAMP. Chemotaxis was stimulated by 10 ng/mL CX3CL1 alone or in the presence of 100μM 8Br-cAMP in NK cells pretreated or not for 1 hour with 100μM NF157. (D) NAD+ and 8Br-cAMP inhibit CX3CL1-elicited NK cytotoxicity against HUVECs. Cells were preincubated with (filled bars) or without (open bars) 100μM NF157 and stimulated with 10 ng/mL CX3CL1 alone in the presence of 100μM NAD+ or 100μM 8Br-cAMP and used for cytotoxicity assay against K562 cells. (A-C) Data are mean ± SD of triplicate cultures from 3 independent experiments. (D) Data are mean ± SD of sextuplicate cultures from 3 independent experiments. All P values were calculated performing Student t test for paired samples.
P2Y11R activation increases cAMPi and mediates ATP-induced inhibition of CX3CL1-elicited chemotaxis and cytotoxicity of NK cells. (A) Effect of NAD+ on CX3CL1-induced NK-cell chemotaxis. Cells were preincubated with or without the indicated inhibitors, stimulated with 10 ng/mL CX3CL1 alone in the presence of the indicated concentrations of NAD+ for 60 minutes and cell migration measured as indicated in “Migration assays.” *P < .05, MRS2159 or NF023 versus NF157. (B) Induction of cAMPi by ATP and NAD+. NK cells were incubated at 37°C for 15 minutes with 100μM ATP or NAD+ in the presence or absence of the indicated inhibitors, and concentration of cAMPi was measured as described in “Intracellular cAMP quantification.” (C) Inhibition of CX3CL1-dependent NK-cell migration by 8Br-cAMP. Chemotaxis was stimulated by 10 ng/mL CX3CL1 alone or in the presence of 100μM 8Br-cAMP in NK cells pretreated or not for 1 hour with 100μM NF157. (D) NAD+ and 8Br-cAMP inhibit CX3CL1-elicited NK cytotoxicity against HUVECs. Cells were preincubated with (filled bars) or without (open bars) 100μM NF157 and stimulated with 10 ng/mL CX3CL1 alone in the presence of 100μM NAD+ or 100μM 8Br-cAMP and used for cytotoxicity assay against K562 cells. (A-C) Data are mean ± SD of triplicate cultures from 3 independent experiments. (D) Data are mean ± SD of sextuplicate cultures from 3 independent experiments. All P values were calculated performing Student t test for paired samples.
Discussion
In this study, we showed that NK cells express a wide array of P2Rs of both P2XR and P2YR subgroups. Pharmacologic analysis showed that extracellular nucleotides activated a Ca2+ signal in NK cells. Although unable to exert direct chemotactic effect on NK cells, ATP induced a marked increase of chemokinesis and displayed opposite regulatory effect on NK-cell chemotactic responses to CX3CL1 and CXCL12 (ie, a potent inhibitory effect on CX3CL1-induced NK-cell migration was exerted by ATP and by its nonhydrolyzable analog ATP-γS but not UTP). On the contrary, stimulation with ATP enhanced NK-cell migration to the lymphoid chemokine CXCL12. In addition, exposure to micromolar concentrations of ATP below the threshold required for P2XR activation significantly impaired the ability of CX3CL1 to stimulate cytotoxicity against ECs.
CX3CL1 plays a central role in NK-EC cross-talk: soluble CX3CL1 is released by activated ECs early during inflammation, recruiting leukocytes bearing its cognate receptor CX3CR1. In addition, CX3CL1 elicits interferon-γ production by NK cells that, in turn, reinforces CX3CL1 expression by ECs.25 Moreover, activated ECs express membrane-bound form of CX3CL1 that functions as an adhesion molecule reinforcing cell-cell interaction. Of note, relatively high concentrations of CX3CL1 are probably present locally in the microenvironment of the NK-EC synapsis where activated endothelial cells produce both soluble and membrane-anchored forms of CX3CL1.
Because of reiterated interaction with extravasating activated leukocytes, ECs are particularly exposed to the risk of cumulating incidental cell damage, especially in the context of excessive inflammation. Although NK/endothelial cell ratios used for in vitro citotoxicity assays might not be exactly reproduced in vivo, smaller NK/EC ratios likely to occur in vivo might be sustained for an amount of time significantly greater than the duration of the in vitro experiment. As a consequence, ECs might cumulate cell injury over time because of the sequential contact with a large number of potentially harmful activated NK cells.
ECs represent a major source of actively secreted ATP,26 pointing it out as an important player in the regulation of NK-EC interaction. In keeping with previous observations, we showed that CX3CL1 can significantly increase cytolytic activity of NK cells toward ECs.9 However, in the presence of ATP, CX3CL1 failed to enhance NK cell–mediated cytolysis of target cells. Similar inhibitory effect was displayed by NAD+ and ATP-γS at 10-fold lower concentration (maximal inhibitory effect of ATP-γS was 10μM vs 100μM for ATP). On the other hand, ATP-dependent inhibition of NK-cell cytotoxicity was not altered by treatment with adenosine deaminase (data not shown), excluding a possible participation of adenosine, as end-product of ATP metabolism, potentially affecting NK cells through the engagement of adenosine receptors. Ruling out ATP metabolites as possible mediators of NK-cell killing inhibition is particularly important because both endothelial and activated NK cells express ectoapyrase (CD39), a membrane ATP diphosphohydrolase that hydrolyzes ATP and ADP into AMP.27,28
NF157, a compound known to inhibit P2Y11R and P2X1R, restored NK-cell responsiveness to CX3CL1 both in terms of chemotaxis and cytolytic activity. A number of observations, however, ruled out the possible involvement the P2X1 receptor in mediating ATP inhibitory effect: (1) although activation of the P2X receptors required greater than 300μM ATP concentrations, the block of CX3CL1 responsiveness occurred at 100μM ATP; (2) α,β-meATP, a potent P2X1R agonist, did not elicit [Ca2+]i increase, or inhibit the ability of CX3CL1 to stimulate NK chemotaxis and cytotoxicity; (3) 3 different P2X1R antagonists, such as MRS2159, NF023, and phenol red, failed to restore NK-cell responsiveness to CX3CL1. On the other hand, we provided evidence pointing out the P2Y11R as key mediator of the inhibitory action exerted by ATP: the inhibition of CX3CL1 capacity to stimulate NK cell was mimicked by NAD+, another physiologic P2Y11R ligand (which is not known to engage P2X1R) and by the ATP analog ATP-γS, which is a better agonist than ATP at the P2Y11R; both ATP and NAD+ induced markedly increased cAMPi in NK cells, and among P2Rs, P2Y11R is the only member known to stimulate adenylyl cyclases, thus increasing cAMPi concentration29 ; ATP and NAD+ failed to increase cAMPi in the presence of NF157 but not P2X1R inhibitors; NK cells exposed to the cell-permeable cAMP analog 8Br-cAMP displayed loss of sensitivity to CX3CL1 similar to that observed after stimulation with ATP or NAD+; and NF157 failed to restore NK-cell responses to CX3CL1 inhibited by 8Br-cAMP. This observation suggests that NK-cell loss of responsiveness to CX3CL1 is secondary to cAMPi elevation, an event downstream to the engagement of P2Y11R by its cognate ligands.
Similarly to what was previously observed in dendritic cells, no intracellular Ca2+ mobilization was induced by BzATP a preferential agonist for P2Y11R, or by ATP-γS despite the expression of discrete amount of the specific mRNA,30 suggesting that in NK and dendritic cells P2Y11R signals almost exclusively through cAMPi as second messenger.
A limitation of this study is the technical impossibility in this setting to demonstrate the role of P2Y11R by a more direct approach, such as receptor gene knockdown. Indeed, in vitro NK-cell viability depends on the presence of IL-2. After stimulation with IL-2, NK cells lost chemotactic response to CX3CL1, making it impossible to assess the effect ATP on chemotaxis. On the other hand, IL-2 strongly stimulated NK-cell cytolytic activity, thus masking any stimulatory effect of CX3CL1 (data not shown).
The activation of the P2Y11R by ATP has been shown to alter the functions of other leukocyte subsets: P2Y11R activation mediated the ATP-dependent inhibition of TNF-α production in human peripheral blood.31 In addition, P2Y11R modulates the maturation of dendritic cells inhibiting TNF-α production32,33 and their ability to migrate in response to the lymphoid chemokine CCL21.30 Furthermore, P2Y11R activation inhibits apoptosis in neutrophils.34
Some pathologic conditions associated with vascular injury can be, at least in part, mediated by NK cells. Heterologous ECs can be targeted by NK-cell cytotoxicity in allograft rejection or graft-versus-host disease. In addition, autologous ECs can become susceptible to NK cell–mediated killing when infected by cytomegalovirus or in subjects with VLS associated with antitumoral therapy based on the administration of IL-2 or IL-2–activated cells. Interestingly, a key role for CX3CL1 in the stimulation of NK cell–mediated vascular injury in VLS has been proposed.9 The observed inhibitory effects exerted by ATP on NK cell–mediated killing of ECs, together with the fact that ECs are able to actively secrete discrete amounts of ATP,26 suggest a potential role for ATP as a physiologic suppressor of incidental cytotoxicity exerted by excessively activated NK cells against ECs. Although the ATP concentration needed to affect NK-cell function in vitro is in the micromolar range, it has to be considered that even very small amounts of ATP secreted by ECs can cause a steep rise of ATP concentration in the microenvironment of the NK-EC synapse. In addition, P2Y11R can be also be engaged by non-nucleotide agonists, such as NAD+24 and NAADP+,35 which might cooperate in vivo with ATP and activate P2Y11R at significantly lower concentrations than those needed in vitro. Moreover, the enhanced NK cell–mediated killing of ECs occurring in the presence of apyrase indicates that the amount of ATP spontaneously released by ECs is sufficient to significantly down-regulate NK cytotoxicity and that the inhibition of killing is not caused by increased extracellular concentration of ATP catabolites, such as adenosine, consistent with what observed by the exogenous addition of the nonhydrolyzable ATP analog ATP-γS and adenosine deaminase.
Noteworthy, as no P2Y11R homolog has been identified in rodents, it is at present unfeasible to study its role on the regulation of NK-ECs cross-talk in vivo.
In conclusion, ECs by secreting ATP, dampen NK-cell cytotoxicity, possibly preventing accidental vascular injury during extravasation of activated NK cells. In addition, our results indicate the P2Y11R as essential mediator of the regulatory effect of ATP on NK-ECs cross-talk. Taken together, our data suggest that selective P2Y11R agonists might be useful in limiting NK cell–mediated vascular injury occurring in various pathologic conditions, such as VLS, allograft rejection, and cytomegalovirus infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof M. E. Mendelsohn for critical review of the manuscript and helpful suggestions.
This work was supported in part by Ricerca Finalizzata Ministero della Salute 2006 Terapia fisica da sola o combinata a terapie specifiche nel trattamento delle cardiovasculopatie, Regione Emilia-Romagna (Progetti di Ricerca Università-Regione Emilia Romagna, Progetto Medicina Rigenerativa, 2007), MIUR (PRIN 2006), the National Research Council of Italy, the Italian Association for Cancer Research, the Italian Space Agency, Telethon of Italy, and the University of Ferrara (local funds).
This paper is dedicated to the memory of Dr Giovanni Gorini.
Authorship
Contribution: S. Gorini and G.C. performed the experiments, analyzed the results, made the figures, and wrote the paper; G. Romagnoli, C.M., D.M., G. Rosano, M.F., S. Gulinelli, S.F., and A.C. performed part of the experiments; and F.D.V., D.F., and A.l.S. designed the research.
Conflict-of-interest disclosure: F.D.V. serves as a consultant for Cordex Pharma Inc and Affectis Pharmaceuticals AG (Germany), companies involved in the development of P2 receptor-based drugs. The remaining authors declare no competing financial interests.
Correspondence: Andrea la Sala, Laboratory of Molecular and Cellular Immunology, IRCCS San Raffaele Pisana, Via dei Bonacolsi, snc 00163 Rome, Italy; e-mail: andrea.lasala@sanraffaele.it.
References
Author notes
S. Gorini and G.C contributed equally to this study.