Adoptive transfer of viral antigen-specific memory T cells can reconstitute antiviral immunity, but in a recent report a majority of virus-specific cytotoxic T-lymphocyte (CTL) lines showed in vitro cross-reactivity against allo-human leukocyte antigen (HLA) molecules as measured by interferon-γ secretion. We therefore reviewed our clinical experience with adoptive transfer of allogeneic hematopoietic stem cell transplantation donor-derived virus-specific CTLs in 153 recipients, including 73 instances where there was an HLA mismatch. There was no de novo acute graft-versus-host disease after infusion, and incidence of graft-versus-host disease reactivation was low and not significantly different in recipients of matched or mismatched CTL. However, we found that virus-specific T cell lines recognized up to 10% of a panel of 44 HLA disparate targets, indicating that virus-specific T cells can have cross-reactivity with HLA-mismatched targets in vitro. These data indicate that the adoptive transfer of partially HLA-mismatched virus-specific CTL is safe despite in vitro recognition of recipient HLA molecules.
Introduction
After stem cell transplantation, there are high morbidity and mortality from viral disease.1 Such complications are commonest where the donor and recipient are partially human leukocyte antigen (HLA)–mismatched or the donor graft has been depleted of mature T lymphocytes to prevent alloreactivity and graft-versus-host disease (GVHD). As a consequence, several investigators have administered donor-derived virus-specific T cells to transplantation recipients to reduce the incidence and severity of posttransplantation viral disease with apparent clinical benefit.2,,,,,,–9 A recent study by Amir et al, however, suggests that transfer of HLA-mismatched virus-specific cytotoxic T-lymphocytes (CTLs) might risk graft-versus-host alloreactions.10 In that study, T-cell lines reactive against Epstein-Barr virus (EBV), cytomegalovirus, varicella zoster virus, and influenza virus were tested against a panel of HLA-typed target cells and target cells transduced with single HLA molecules.10 Remarkably, 80% of virus-specific T-cell lines and 45% of virus-specific T-cell clones derived therefrom were cross-reactive against allo-HLA molecules, as measured by γ-interferon secretion.10 This cross-reactivity was observed in both CD8+ and CD4+ T-cell clones, being directed primarily against HLA class I and II antigens, respectively. These observations raise the concern that virus-specific T cells might mediate graft rejection or GVHD when administered to HLA class I or II mismatched recipients.10
Notwithstanding the apparently high level of cross-reactivity in the in vitro assays reported by Amir et al,10 there are no data to suggest that cross-reactivity of virus-specific T cells with HLA specificities leads to clinical complications.3,,,,,–9 None of these studies, however, formally dissected responses in recipients who had received HLA partially mismatched virus-specific CTLs, or examined whether the observed lack of any GVHD was simply the result of fortuitous absence of alloreactivity in the administered lines.
We now report that, in 73 recipients of virus-specific CTLs from an HLA-mismatched donor, we have not observed GVHD associated with the cell infusion. In 4 patients, the alloreactivity of infused lines was characterized in an in vitro assay against a T cell–antigen-presenting cell (APC) panel. Our data confirm the presence of in vitro allo-HLA reactivity in infused virus-specific T cells but do not support the conclusion that such alloreactive CTLs can cause GVHD in vivo.
Methods
Patient details
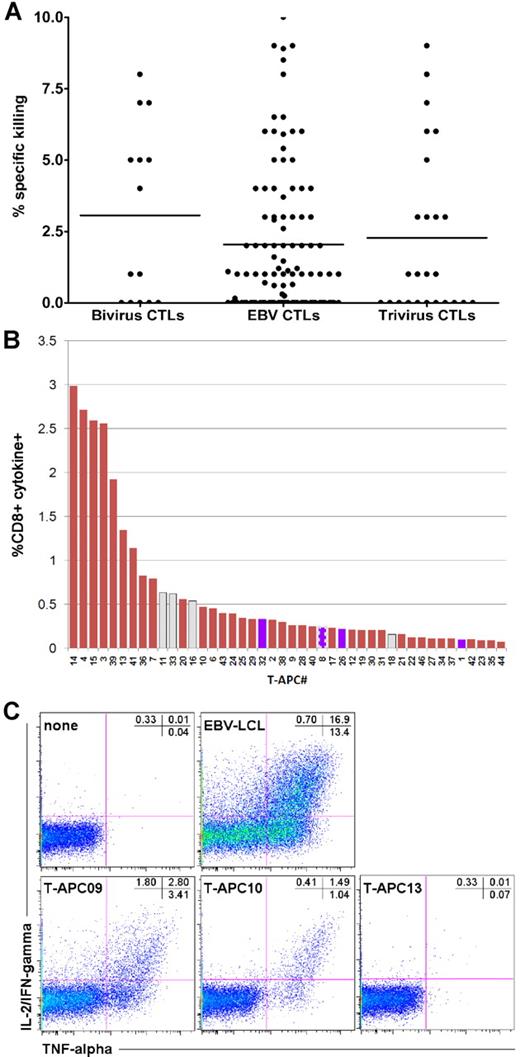
Hematopoietic stem cell transplantation recipients were treated on studies of donor-derived EBV-specific CTLs,2 bivirus CTLs specific for adenovirus and EBV,4 and trivirus CTLs specific for cytomegalovirus, adenovirus, and EBV.3 All studies were approved by the Food and Drug Administration and the Institutional Review Board at Baylor College of Medicine. Clinical details and results of the studies have been previously reported.2,–4 In these studies, one release criterion to exclude alloreactivity was that killing of recipient phytohemagglutinin blasts by the infused CTL line should be less than 10%11 (with < 2% of manufactured lines failing to meet this criterion), and data from the 3 studies are shown in Figure 1A. A total of 73 of the 153 subjects had a donor that was mismatched at 1 or more HLA antigens.
In vitro assay of alloreactivity
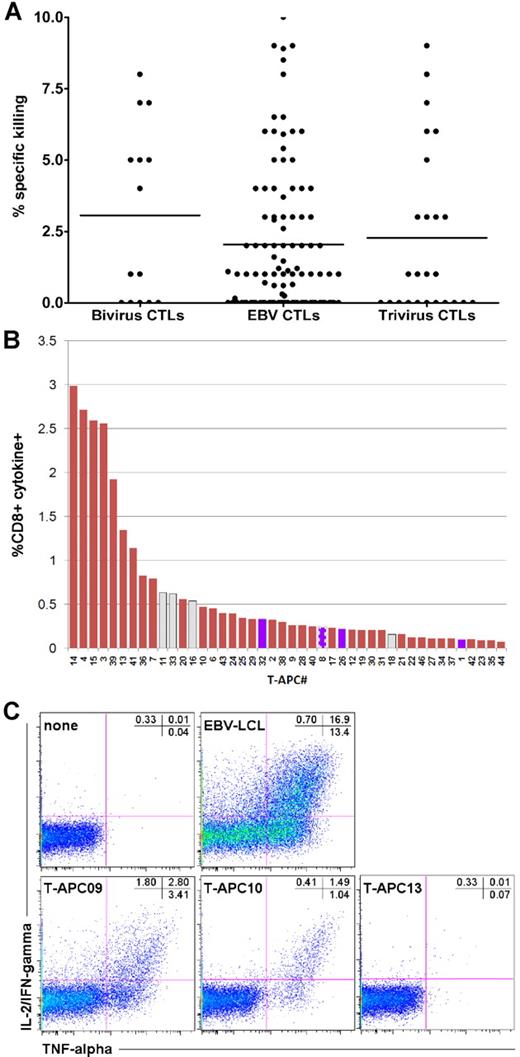
Four CTL lines from the adenovirus/EBV CTL study underwent analysis for alloreactivity on studies approved by Institutional Review Boards at the National Institutes of Health and Baylor. Activated T cells were generated as described12 under National Heart, Lung, and Blood Institute Institutional Review Board-approved protocols, and served as APCs (T-APCs). A panel of 44 T-APCs was composed to cover the most frequent HLA class I and II alleles (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For the detection of allogeneic targets, the virus-specific T-cell lines were labeled with carboxyfluorescein succinimidyl ester13 and stimulated with unlabeled T-APCs (supplemental Table 1) or left unstimulated.12 After 6 hours, the cells were processed as outlined in detail in supplemental Methods. Responder cells were identified in the carboxyfluorescein succinimidyl ester-positive population by cells that produced both tumor necrosis factor-α (TNF-α) and interleukin-2 (IL-2)/interferon-γ (IFN-γ) because the background signal for this cytokine combination (ie, the proportion of T cells producing TNF-α and IL-2/IFN-γ cultured for 6 hours with secretion inhibitors alone) was lower compared with TNF-α or IL-2/IFN-γ separately as can be seen in Figure 1C. The respective T-cell lines cultured under the same conditions but in the absence of T-APCs were used as negative controls, and the proportion of cytokine-producing cells was subtracted from the panel analysis to arrive at the net allo-HLA response (supplemental Methods).
Results and discussion
GVHD in mismatched patients
Of the 73 recipients of HLA-mismatched virus-specific CTLs, 34 received EBV-specific CTLs from an unrelated donor mismatched at 1 or more HLA antigens, whereas 13 received EBV-specific CTLs produced from an HLA haploidentical family member. Five received bivirus-specific CTLs from unrelated donors mismatched at 1 or 2 of 10 HLA antigens and 6 from haploidentical donors. Six subjects received trivirus CTLs from an unrelated donor mismatched at 1 to 3 of 10 antigens and 9 from a haploidentical family member. The overall incidence of acute GVHD was 6.5%, with all episodes representing reactivations and no significant difference in the incidence between recipients of matched or mismatched CTLs (Table 1). Because GVHD only occurred in patients with previous episodes of the disease, the absence of de novo GVHD after CTL infusions and the lack of correlation with the degree of mismatching of the CTLs indicated that mismatched CTLs did not induce GVHD.
In vitro alloreactivity
To exclude the possibility that the observed absence of GvHD was the result of the fortuitous choice of CTLs lacking recipient-specific alloreactivity, we analyzed the HLA reactivity of 4 infused bivirus-specific lines. All CTLs responded to a number of T-APCs (Figure 1B-C; supplemental Figure 1; supplemental Table 2). Some T-APCs were recognized strongly, and strong alloreactivity appeared to be confined to the CD4+ T-cell subset (eg, CTL C2910 and C3000; supplemental Figure 2), whereas most stimulators induced only weak or undetectable cytokine signals (supplemental Figure 1). The proportion of stimulators recognized ranged from broad (CTLs C2910, C3000, and C3311) to restricted (C3183), suggesting polyclonal versus oligoclonal responses. We then determined whether the CTLs recognized T-APCs expressing the HLA alleles of the recipient (supplemental Table 3). In the 4 lines tested, virus-specific CD4+ and CD8+ T cells displayed moderate reactivity with 1 to 5 T-APCs expressing the recipient's HLA allele (Figure 1B; supplemental Figure 1).
Alloreactivity of infused CTLs. Before infusing the donor CTLs, we characterized their cytotoxicity against phytohemagglutinin blasts obtained from the transplantation recipient in a standard chromium release assay.11 The release criterion was that cytotoxicity should be less than 10%. (A) Mean cytotoxicity was 2.07% for EBV CTLs, 2.24% for trivirus CTLs, and 3.07% for bivirus CTLs. Each symbol represents a cell line infused into a single subject. (B) The response of CD8+ T cells in CTL line C3183, to the panel of T-APCs. Only cells producing both IL-2/IFN-γ and TNF-α were scored positive, and the responses are ranked according to their frequency. CD8+ T cells responding to APCs that expressed an HLA class I allele shared with the recipient of this cell line are indicated as blue bars. Because allo-HLA reactive CD8+ T cells can recognize HLA class II-presented antigens, we have displayed their reactivities with T-APCs expressing a recipient-matching HLA class II mismatch as gray bars. One T-APC expressed both an HLA class I and II mismatch shared with the recipient of C3183; the response to this APC is represented as a blue bar with bold striped gray border. (C) The response of CTL line C2910 to autologous EBV-lymphoblastoid cell line (LCL) and 3 representative T-APCs, plus the negative control: CTL line without APCs.
Alloreactivity of infused CTLs. Before infusing the donor CTLs, we characterized their cytotoxicity against phytohemagglutinin blasts obtained from the transplantation recipient in a standard chromium release assay.11 The release criterion was that cytotoxicity should be less than 10%. (A) Mean cytotoxicity was 2.07% for EBV CTLs, 2.24% for trivirus CTLs, and 3.07% for bivirus CTLs. Each symbol represents a cell line infused into a single subject. (B) The response of CD8+ T cells in CTL line C3183, to the panel of T-APCs. Only cells producing both IL-2/IFN-γ and TNF-α were scored positive, and the responses are ranked according to their frequency. CD8+ T cells responding to APCs that expressed an HLA class I allele shared with the recipient of this cell line are indicated as blue bars. Because allo-HLA reactive CD8+ T cells can recognize HLA class II-presented antigens, we have displayed their reactivities with T-APCs expressing a recipient-matching HLA class II mismatch as gray bars. One T-APC expressed both an HLA class I and II mismatch shared with the recipient of C3183; the response to this APC is represented as a blue bar with bold striped gray border. (C) The response of CTL line C2910 to autologous EBV-lymphoblastoid cell line (LCL) and 3 representative T-APCs, plus the negative control: CTL line without APCs.
Lack of in vitro/in vivo correlation
Because most patients were off immunosuppressive treatment at the time of T-cell infusion, other explanations must be sought to explain the lack of GVHD in recipients of T-cell lines recognizing the HLA type of the recipient. It is possible that the lack of reactivity with GVHD-susceptible tissues in the recipient could be explained by a discordance in antigen expression between the T-APCs and GVHD targets, as might be the case in the study by Amir et al.10 Although it is possible that GVHD-reactive T-cell lines had only a limited capacity to survive and expand in vivo,14 our gene-marking studies suggest that these infused virus-specific cells are long lived.2 Because GVHD may be primarily mediated by naive T cells,15 the predominance of memory-effector cells in these infusions may have been protective. Alternatively, the polarity of the infused T cells may have protected against GVHD,16 but the Th1/Th2 characteristics of these lines were not studied. Irrespective of the ultimate explanation, our conclusion from the data is that in vitro alloreactivity of virus-specific T-cell lines against hematopoietic APCs does not correlate with the risk of developing GVHD and that alloreactive T-cell lines can be safely infused into both major histocompatibility complex class I and II mismatched recipients.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the clinicians involved in care of these patients, the staff in the GMP facilities who manufactured cells, and all the research staff who collected data.
This work was supported by the National Heart, Lung, and Blood Institute (intramural grant; J.J.M., A.J.B.), the National Institutes of Health (grants RO1CA061384, U54HL081007, P01CA094237, and P50CA126752), and the Leukemia & Lymphoma Society (Specialized Center of Research grant). H.E.H. is supported by a Dan L. Duncan Chair. M.K.B. is supported by a Fayez Sarofim Chair.
National Institutes of Health
Authorship
Contribution: J.J.M. performed laboratory studies of alloreactivity, interpreted data, and contributed to writing the manuscript; A.M.L. developed the methodology for multivirus specific CTLs, performed laboratory analyses, and reviewed the manuscript; C.M.B. was principal investigator of the multivirus CTL studies and reviewed the manuscript; M.F.Q. and D.A.P. assisted in large-scale screening experiments; C.M.R. developed the standard operating procedures for CTL production, developed the clinical trials, and reviewed the manuscript; M.K.B. developed the clinical trials and reviewed the manuscript; A.J.B. interpreted data from studies of alloreactivity and contributed to writing the manuscript; and H.E.H. developed the clinical trials, was principal investigator of the EBV CTL study, analyzed the data, and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helen E. Heslop, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1630, Houston, TX 77030; e-mail: hheslop@bcm.edu.