Abstract
Current induction schemes directing hematopoietic differentiation of human embryonic stem cells (hESCs) are not well defined to mimic the sequential stages of hematopoietic development in vivo. Here, we report a 3-stage method to direct differentiation of hESCs toward hematopoietic progenitors in chemically defined mediums. In the first 2 stages, we efficiently generated T-positive primitive streak/mesendoderm cells and kinase domain receptor–positive (KDR+) platelet-derived growth factor receptor α–negative (PDGFRα−) hemato-vascular precursors sequentially. In the third stage, we found that cells in a spontaneous differentiation condition mainly formed erythroid colonies. Addition of all-trans retinoic acid (RA) greatly enhanced generation of hematopoietic progenitors in this stage while suppressing erythroid development. The RA-treated cells highly expressed definitive hematopoietic genes, formed large numbers of multilineage and myeloid colonies, and gave rise to greater than 45% CD45+ hematopoietic cells. When hematopoietic progenitors were selected with CD34 and C-Kit, greater than 95% CD45+ hematopoietic cells could be generated. In addition, we found that endogenous RA signaling at the second stage was required for vascular endothelial growth factor/basic fibroblast growth factor–induced hemato-vascular specification, whereas exogenously applied RA efficiently induced KDR−PDGFRα+ paraxial mesoderm cells. Our study suggests that RA signaling plays diverse roles in human mesoderm and hematopoietic development.
Introduction
Human embryonic stem cells (hESCs) have the capacity to self-renew indefinitely and to differentiate into almost all cell types.1 These unique characteristics mean that hESCs can provide a model of the early hematopoietic development of the human embryo and can be applied to hematopoietic cells transplantation. Several works studying hematopoietic development from hESCs have been reported (reviewed in Murry and Keller2 ). Despite the generation of hematopoietic progenitor/stem cells, the procedures these works applied are not well defined to mimic the process of in vivo hematopoietic development. Moreover, most of these works involve coculturing with feeder cells or induction with serum-containing mediums, making it difficult to study the molecular mechanisms regulating the cell fate decision at each stage. The ideal strategy for the derivation of hematopoietic cells from hESCs should be chemically defined and stepwise to mimic hematopoietic development in vivo.
During mouse embryogenesis, hematopoietic ontogeny mainly involves the sequential formation of mesoderm, hemato-vascular precursors, and hematopoietic cells (reviewed in Orkin and Zon3 and in Dzierzak and Speck4 ). After the establishment of 3 germ layers during gastrulation, 2 types of hemato-vascular precursors are identified. At mid-streak stage in mouse embryos, a subset of mesoderm precursors in the posterior primitive streak have both hematopoietic (primitive and definitive) and vascular potentials—presumably hemangioblast.5 The primitive hematopoietic cells mainly contain large nucleated erythroid cells that express embryonic globin chains, which distinguishes them from definitive (adult) hematopoietic cells. Another type of hematopoietic precursors possessing the characteristic of endothelial cells has been identified in the aorta-gonad-mesonephros (AGM) region, the yolk sac, and the placenta, which is termed hemogenic endothelium.6-8 Both cell types have been identified in differentiated mouse and hESCs.9-13 Although the relationship between hemangioblasts and hemogenic endothelium has not been determined, a recent study has suggested that hemangioblasts further generate hematopoietic progenitors through a transient hemogenic endothelium intermediate.14 At a later stage, hematopoietic stem/progenitor cells bud from the hemogenic endothelium.8 Several signaling pathways have been suggested to regulate this event, including Notch,15,16 bone morphogenetic protein (BMP)17 and in particular, all-trans retinoic acid (RA).18-21
Here, by mimicking the in vivo hematopoietic development, we developed a 3-stage chemically defined method to direct the differentiation of hematopoietic progenitors from hESCs. Our results show that hematopoietic differentiation from hESCs can mimic the stages of hematopoietic development in vivo. Moreover, we found that in the second stage, endogenous RA signaling was required for vascular endothelial growth factor/basic fibroblast growth factor (VEGF/bFGF)–induced hemato-vascular specification from primitive streak/mesendoderm cells, and the exogenously applied RA efficiently induced paraxial mesoderm cells. In the third stage, RA enhanced the generation of hematopoietic progenitors and decreased erythroid development from hemato-vascular precursors.
Methods
Culture and differentiation of hESCs
H1 and H9 hESC lines (Wicell Research Institute) were maintained on irradiated mouse embryonic fibroblast feeder cells with Dulbecco modified Eagle medium nutrient mixture F12 supplemented with 20% knockout serum replacement, 1mM l-glutamine, 1% nonessential amino acids, 1% penicillin/streptomycin, 0.1mM β-mercaptoethanol (all from Gibco), and 8 ng/mL human bFGF (PeproTech) at 37°C and 5% CO2 in a humidified atmosphere. Cells were split 1:3 every 5 days with 1 mg/mL collagenase IV (Gibco). Unless otherwise stated, all experiments were conducted with H1 cells.
For hematopoietic differentiation, 70% confluent hESC colonies were washed with phosphate buffered saline (PBS) and incubated with 1 mg/mL Dispase (Gibco) for 15 minutes. The colonies were gently pipetted and detached from the feeder layer and transferred to a 15-mL conical tube. Colonies were allowed to settle for 10 minutes and washed twice in PBS (Gibco). Next, the cell aggregates were cultured in suspension on ultralow attachment 6-well plates (Costar) in basic medium (75% Iscove modified Dulbecco medium [IMDM], 25% F12, 0.05% bovine albumin fraction V, 1% penicillin/streptomycin; all from Gibco) supplemented with 50 ng/mL human BMP4 (PeproTech) and 100 ng/mL mouse Wnt3a (R&D Systems) or as indicated. At day 1.5, embryoid bodies (EBs) were transferred to a 15-mL conical tube, settled for 10 minutes, and washed with PBS. Then EBs were cultured on ultralow attachment 6-well plates again in stage 2 medium consisting of basic medium supplemented with 1% insulin-transferrin-selenium-A (ITS; Gibco), 0.5mM ascorbic acid (Sigma-Aldrich), and 0.45mM monothioglycerol (Sigma-Aldrich). VEGF (100 ng/mL human; PeproTech), bFGF (100 ng/mL), or indicated induction factors were added at stage 2. The medium was changed at day 3, and at day 4 EBs were changed to stage 3 medium (basic medium supplemented with 0.5mM ascorbic acid and 0.45mM monothioglycerol, 0.5% N2 supplements, and 1% B27 supplements without vitamin A; both from Gibco)22 supplemented with 2μM all-trans RA (Sigma-Aldrich) or LE540 (Wako). Stage 3 medium was changed every day.
For generating CD45+ cells, indicated cells were cultured for 7 days on ultralow attachment 24-well plates at 40 000 cells/well in StemSpan serum-free expansion medium (StemCell Technologies) supplemented with 10% fetal bovine serum (FBS; Gibco), 50 ng/mL human stem cell factor (SCF), 50 ng/mL human Flt-3 ligand (Flt-3L), 10 ng/mL human interleukin-3 (IL-3), and 10 ng/mL human IL-6 (all from PeproTech). Half of the medium was changed every 2 days.
Cell sorting
The selection of CD34+/− cells was performed with a CD34 MicroBead Kit (Miltenyi Biotec) according to the manufacturer's instructions. Briefly, day 7 EB cells were dissociated with trypsin/EDTA (ethylenediaminetetraacetic acid), filtered through a 40-μm cell strainer (BD Falcon), and resuspended in 300 μL of PBS containing 3% FBS. These cells were incubated with CD34 microbeads for 30 minutes at 4°C, washed with PBS/3% FBS, and passed through an MS column (Miltenyi Biotec). For CD34− cell selection, dropped cells were collected and passed through a new MS+ column again. CD34+ cells retained on the column were eluted with PBS containing 3% FBS. For second labeling, CD34+ cells were resuspended in PBS containing 3% FBS and incubated with allophycocyanin-conjugated anti–C-Kit or allophycocyanin-conjugated isotype control (both from BD Biosciences) for 30 minutes at 4°C. After 3 washings, the cells were sorted with a MoFlo high-performance cell sorter (Cytomation).
For selection of cord blood–derived CD34+ cells, mononuclear cells were first isolated from umbilical cord blood with Histopaque-1077 (Sigma-Aldrich). Then the cells were incubated with CD34 microbeads, washed, and separated on a MS column.
Colony-forming cell assays
Single cells of indicated numbers in 0.1 mL of IMDM with 3% FBS were mixed with 1 mL of MethoCult GF+ 4435 (StemCell Technologies). Then the mixture was transferred to 35-mm dishes (StemCell Technologies). Cells were incubated for 14 days and, after 14 days, 35-mm dishes were plated to 60-mm gridded dishes (StemCell Technologies) for scoring. Each type of colony-forming unit (CFU) was characterized by its structure. Each sample was in triplicate. Images were recorded with the use of NIS-Elements F 2.30 in the Nikon ECLIPSE TE2000-U microscope system and processed with Photoshop CS software (Adobe Systems).
For drug toxicity sensitivity assay, 5000 hESC-derived CD34+ C-Kit+ hematopoietic progenitors or 2000 cord blood CD34+ cells in 0.1 mL of IMDM with 3% FBS were mixed with 1 mL of MethoCult H4001 (StemCell Technologies). Dimethyl sulfoxide (DMSO) or indicated chemicals were added to the mixture. Doxorubicin and paclitaxel (both from Sigma-Aldrich) dilutions were prepared in 1000× the final dilution in DMSO to obtain the final concentrations of 0.2, 1, 5, 20, 100, and 500 ng/mL. After culture for 14 days, colony numbers were scored and normalized to DMSO samples. Each sample was in triplicate.
Formation and expansion of blast colonies
A total of 20 000 single day 4 EB cells in 0.1 mL of IMDM were mixed with 1 mL of MethoCult SF H4436 (StemCell Technologies) supplemented with 50 ng/mL human VEGF and 50 ng/mL human BMP4.11 Then the mixture was transferred to 35-mm dishes. Cells were incubated for 6 days and, after 6 days, 35-mm dishes were plated to 60-mm gridded dishes for scoring.
For blast colony expansion, single colonies were picked and transferred into 96-well plates coated with Matrigel (growth factor reduced; BD Biosciences) that contained IMDM supplemented with 10% FBS, 10% horse serum (Invitrogen), 1mM l-glutamine, 4 × 104M monothioglycerol, 1% ITS, 5 ng/mL human bFGF, 10 ng/mL human VEGF, 100 ng/mL human SCF, 20 ng/mL human IL-6, 2 U/mL human erythropoietin (R&D systems), 5 ng/mL human IL-11 (PeproTech), and 25 ng/mL human insulin-like growth factor 1 (PeproTech).12 After 6 days of culture, nonadherent cells were gently harvest for flow cytometric analysis. The adherent cells were examined for the expression of endothelial cell markers.
Flow cytometric and reverse transcription–PCR/real-time PCR analyses
Flow cytometry and reverse transcription polymerase chain reaction (PCR)/real-time PCR analyses were performed with the default conditions. Detailed information is described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Staged hematopoietic differentiation of hESCs
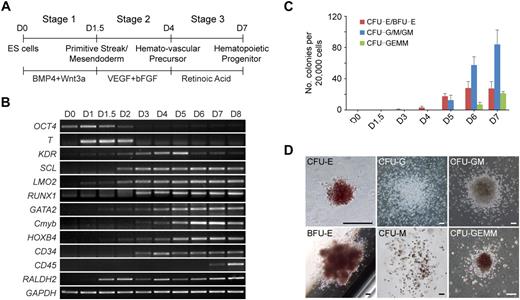
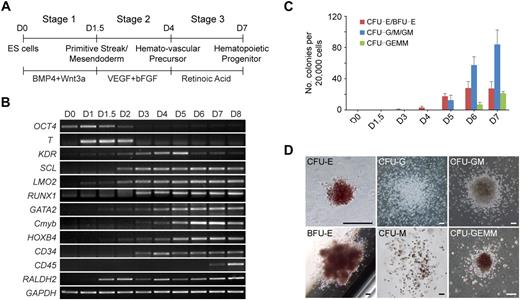
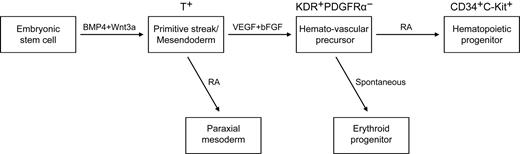
To study the regulation of hematopoietic development, we first developed a chemically defined 3-stage procedure to direct the differentiation of hESCs to the hematopoietic lineage (Figure 1A; supplemental Figure 1). The procedure involved the initiation of primitive streak/mesendoderm cells with BMP4 and Wnt3a for the first 1.5 days, the specification to hemato-vascular precursor with VEGF and bFGF for 2.5 days, the commitment and expansion of hematopoietic progenitors with RA treatment for another 3 days.
Staged differentiation of hematopoietic progenitors from hESCs. (A) Schematic for the 3-stage hematopoietic differentiation protocol of hESCs. (B) Gene expression changes of EB cells at different stages of development were analyzed by reverse transcription PCR. (C) Development of CFCs during hematopoietic differentiation. (D) Photographs of different hematopoietic colony types derived from stage-3 EB cells. CFU-E indicates erythroid CFU; BFU-E, erythroid burst-forming unit; CFU-GEMM, granulocyte/erythroid/macrophage/megakaryocyte CFU. Images were captured under culture mediums of the cells. The image of CFU-E is shown at 40× original magnification, and the other images are shown at 100× original magnification. Images were recorded with NIS-Elements F 2.30 in the Nikon ECLIPSE TE2000-U microscope system and processed with the Adobe Photoshop CS software. Scale bar, 200 μm. Error bars indicate mean ± SEM (n = 3).
Staged differentiation of hematopoietic progenitors from hESCs. (A) Schematic for the 3-stage hematopoietic differentiation protocol of hESCs. (B) Gene expression changes of EB cells at different stages of development were analyzed by reverse transcription PCR. (C) Development of CFCs during hematopoietic differentiation. (D) Photographs of different hematopoietic colony types derived from stage-3 EB cells. CFU-E indicates erythroid CFU; BFU-E, erythroid burst-forming unit; CFU-GEMM, granulocyte/erythroid/macrophage/megakaryocyte CFU. Images were captured under culture mediums of the cells. The image of CFU-E is shown at 40× original magnification, and the other images are shown at 100× original magnification. Images were recorded with NIS-Elements F 2.30 in the Nikon ECLIPSE TE2000-U microscope system and processed with the Adobe Photoshop CS software. Scale bar, 200 μm. Error bars indicate mean ± SEM (n = 3).
Gene expression analysis showed that the developing EBs induced with this staged method mimicked the process of in vivo hematopoietic development (Figure 1B). Expression of the primitive streak/mesendoderm gene T, was observed on days 1 to 2 and peaked at the end of stage 1 (day 1.5). During stage 2 (days 1.5-4), genes representative of the hematopoietic program began to be expressed, including KDR, SCL, LMO2, GATA2, RUNX1, Cmyb, HOXB4, and CD34. All of these genes continued to be expressed throughout the subsequent days of differentiation. However, the expression of KDR, which is not found in late hematopoietic progenitors,23 peaked at day 5, then decreased during stage 3 (days 4-7). In contrast to KDR, the GATA2, Cmyb, and HOXB4 genes were up-regulated from day 5. Meanwhile, the myeloid and mixed colony-forming cells (CFCs) also greatly increased at stage 3 (Figure 1C). The exclusive hematopoietic marker CD45 appeared at day 7, and its expression increased rapidly. Because the strong expression of CD45 indicates maturation of myeloid lineage progenitors,24 we ended stage 3 at day 7. After 7 days of development, the EB cells generated all types of hematopoietic colonies in colony-forming assays, including erythroid burst-forming unit, erythroid CFU, granulocyte CFU (CFU-G), macrophage CFU (CFU-M), granulocyte/macrophage CFU (CFU-GM), and granulocyte/erythroid/macrophage/megakaryocyte CFU (Figure 1D).
Initiation of primitive streak/mesendoderm differentiation from hESCs by BMP4 and Wnt3a
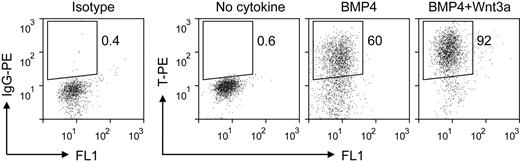
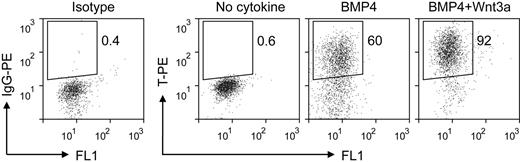
The first stage of our induction procedure is primitive streak/mesendoderm initiation. We previously have reported that the short-term treatment with BMP4 initiates the differentiation of mesoderm lineage from hESCs.25 Here, we optimized this step of EB differentiation system by adding Wnt3a for the first 1.5 days. Analysis of the expression of the primitive streak/mesendoderm marker T by intracellular flow cytometric assay showed that the combination of BMP4 and Wnt3a induced greater than 90% T-positive cells, compared with 60% when treated with BMP4 alone (Figure 2). The low expression levels of primitive streak/mesendoderm and endoderm markers FOXA2 and GSC under this condition (supplemental Figure 2) suggested that this T-positive population was posterior primitive streak cells. The synergy between Wnt3a and BMP4 is consistent with previous studies that Wnt signaling plays a critical role in mesoderm and hematopoietic development in both mouse and human ESC differentiation.22,26-28 In the spontaneous differentiated EBs, only a few cells expressed T, and at low levels (Figure 2 no cytokine). These results show that the combination of BMP4 and Wnt3a efficiently initiates primitive streak/mesendoderm differentiation in the EB system.
Intracellular flow cytometric analysis for T expression in EB cells treated with different cytokines for the first 1.5 days. IgG-PE indicates immunoglobulin G phycoerythrin.
Intracellular flow cytometric analysis for T expression in EB cells treated with different cytokines for the first 1.5 days. IgG-PE indicates immunoglobulin G phycoerythrin.
RA signaling regulated mesoderm subset specification
The second stage was mesoderm subset specification. We used the expression patterns of kinase domain receptor (KDR) and platelet-derived growth factor receptor α (PDGFRα) determined by flow cytometric analysis to assess the development of the hemato-vascular lineage and the paraxial mesoderm.29
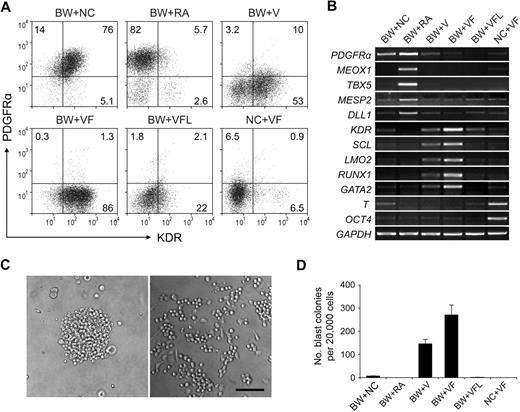
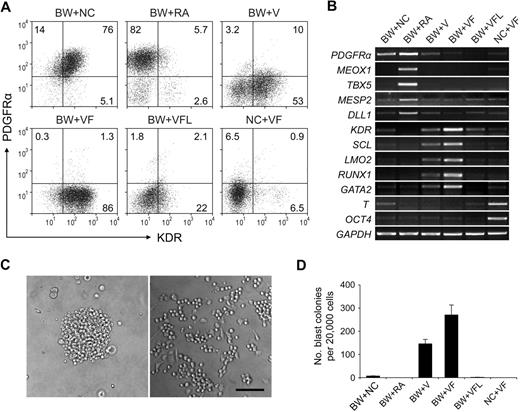
When day 1.5 EBs were cultured for another 2.5 days in the absence of induction factors, most of the cells coexpressed KDR and PDGFRα (Figure 3A BW+NC). Because there was still some expression of T (Figure 3B lane BW+NC), these cell populations may represent mesoderm cells in an early immature stage, which have been suggested in an earlier study that used mouse ESCs.29
Subset specification of primitive streak/mesendoderm cells. BMP4 and Wnt3a-induced EBs (day 1.5) were cultured with different induction factors for 2.5 days. Numbers represent percentage of total cells. The characteristics of day 4 EBs were analyzed by flow cytometry with KDR and PDGFRα (A) and by reverse transcription PCR (B). (C) Images of blast colony derived from day 4 EB cells (left) and hematopoietic and endothelial cells derived from this single blast colony (right). Images were captured under culture mediums of the cells and are shown at 100× original magnification. (D) Hematopoietic and endothelial potentials of day 4 EB cells under different conditions were assessed by blast colony-forming assay. Error bars indicate mean ± SEM (n = 3). Each sample was in triplicate. BW indicates BMP4 and Wnt3a; NC, no cytokine; V, VEGF; F, bFGF; L, LE540. Images were recorded with the use of NIS-Elements F 2.30 in the Nikon ECLIPSE TE2000-U microscope system and processed with the Adobe Photoshop CS software. Scale bar, 200 μm.
Subset specification of primitive streak/mesendoderm cells. BMP4 and Wnt3a-induced EBs (day 1.5) were cultured with different induction factors for 2.5 days. Numbers represent percentage of total cells. The characteristics of day 4 EBs were analyzed by flow cytometry with KDR and PDGFRα (A) and by reverse transcription PCR (B). (C) Images of blast colony derived from day 4 EB cells (left) and hematopoietic and endothelial cells derived from this single blast colony (right). Images were captured under culture mediums of the cells and are shown at 100× original magnification. (D) Hematopoietic and endothelial potentials of day 4 EB cells under different conditions were assessed by blast colony-forming assay. Error bars indicate mean ± SEM (n = 3). Each sample was in triplicate. BW indicates BMP4 and Wnt3a; NC, no cytokine; V, VEGF; F, bFGF; L, LE540. Images were recorded with the use of NIS-Elements F 2.30 in the Nikon ECLIPSE TE2000-U microscope system and processed with the Adobe Photoshop CS software. Scale bar, 200 μm.
Because RA signaling has been suggested to regulate hemato-vascular development,19,30 we next sought to test the effect of RA at this stage. Unexpectedly, when exogenous RA was added, a KDR− PDGFRα+ cell population was efficiently generated (Figure 3A BW+RA). Because this profile marks the paraxial mesoderm,29 we examined the expression of paraxial mesoderm markers MEOX1, TBX5, MESP2, and DLL1 with the use of reverse transcription PCR. The high-level expression of these genes in RA-treated cells suggested that RA induced paraxial mesoderm specification from primitive streak/mesendoderm cells (Figure 3B lane BW+RA) and that this induction effect was dose dependent (supplemental Figure 3).
We next used VEGF and bFGF, 2 factors known to be involved in hemato-vascular development,31-33 to induce the development of hemato-vascular precursors at this stage. When VEGF was added to the culture alone, a KDR+PDGFRα− population was readily generated (Figure 3A BW+V). When combined with bFGF, this induction effect increased dramatically, because greater than 85% cells became KDR+PDGFRα− (Figure 3A BW+VF). With this condition, we also observed the highest expression levels of the hemato-vascular precursor markers, KDR, LMO2, SCL, GATA2, and RUNX1 (Figure 3B lane BW+VF). In addition, the VEGF/bFGF-induced cells generated a large number of blast colonies (Figure 3C-D). These individual colonies could give rise to both nonadherent hematopoietic cells and adherent endothelial cells (Figure 3C; supplemental Figure 4), which was consistent with previous results from other studies,11,12,34 further confirming the efficacy of the VEGF/bFGF combination for specification of the hemato-vascular lineage from primitive streak/mesendoderm cells. The blast CFCs were enriched in KDR+PDGFRα− cells (supplemental Figure 5). Most KDR+ cells at day 4 did not coexpress hESC marker SSEA-4 and TRA-1-60 (supplemental Figure 4), which excluded the presence of undifferentiated cells in this cell population. Cells that did not undergo BMP4 and Wnt3a induction in stage 1 retained expression of the stem cell marker OCT4 and exhibited strong expression of T (Figure 3B lane NC+VF), suggesting that these cells were still undifferentiated and at early mesoderm stages.
Because the enzyme responsible for all RA activity in early mouse embryo, RALDH2,35,36 was expressed from day 1.5 in our differentiation system (Figure 1B), we speculated that endogenous RA secreted by the cells in culture plays a role in the mesoderm specification process. To assess whether this endogenous RA signaling is required in VEGF/bFGF-induced hemato-vascular lineage specification, the pan-RA receptor antagonist LE540 (4μM) was added to the culture. At this concentration, RA-induced expression of the paraxial mesoderm marker MEOX1 in day 4 EB cells was completely suppressed (supplemental Figure 3). When LE540 was added to the VEGF/bFGF culture, the generation of the KDR+PDGFRα− population decreased dramatically (Figure 3A BW+VFL), and expression levels of the hemato-vascular precursor genes and the capacity to generate blast colonies also decreased (Figure 3B,D). These results suggest that, although exogenous applied RA induces paraxial mesoderm specification, endogenous RA signaling is required for hemato-vascular specification from primitive streak/mesendoderm.
RA enhances the generation of hematopoietic progenitors from hemato-vascular precursors
To evaluate the differentiation of hematopoietic progenitors in stage 3, we used CFC assays to assess the hematopoietic potential of cells under different conditions.
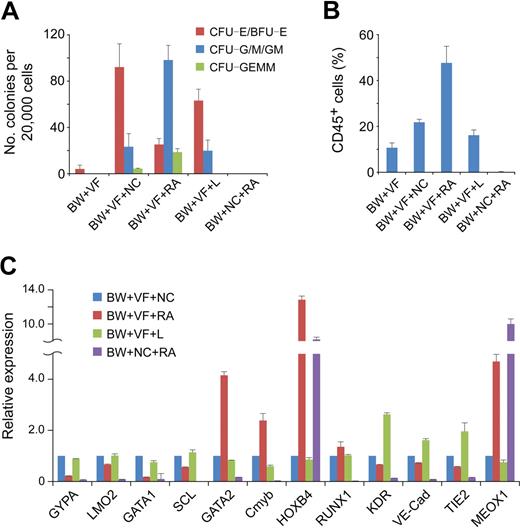
When plated in methylcellulose, stage 2 hemato-vascular precursors at day 4 (BW+VF) generated a small number of erythroid colonies (Figure 4A). After 3 days of spontaneous differentiation (BW+VF+NC), a large number of colonies were obtained from day 7 cells. However, approximately 70% of the colonies were erythroid lineage CFCs, and the numbers of multilineage colonies (granulocyte/erythroid/macrophage/megakaryocyte CFU) and myeloid colonies (CFU-G/M/GM) were relatively low (Figure 4A). Real-time PCR analysis showed that cells under this spontaneous differentiation condition expressed high levels of the erythroid genes GYPA, SCL, LMO2, and GATA1 (Figure 4C). These results suggest a robust erythroid development of stage 2 hemato-vascular precursors under spontaneous differentiation.
Effect of RA on hematopoietic progenitor commitment from hemato-vascular precursors. Stage 2 EBs (BW+VF) were cultured for 3 days in the presence of different induction factors. BW indicates BMP4 and Wnt3a; NC, no cytokine; V, VEGF; F, bFGF; L, LE540. (A) Hematopoietic potential of day 7 EB cells under different conditions were assessed by methylcellulose hematopoietic colony assay. Error bars indicate mean ± SEM (n = 4). Each sample was in triplicate. (B) Day 7 EB cells generated under different conditions were cultured as aggregates for 7 days with serum-free expansion medium supplemented with 10% FBS, SCF, FLT-3L, IL-3, and IL-6. Expression of CD45 was determined by flow cytometric analysis. Errors bars indicate mean ± SEM (n = 4). (C) Gene expression analysis by real-time PCR of day 7 EB cells under different conditions. The relative gene expression was normalized to the cells in culture without any induction factors in stage 3. Similar results were produced by 3 independent experiments with 3 parallel samples. The data were from one experiment, and data represent mean ± SEM.
Effect of RA on hematopoietic progenitor commitment from hemato-vascular precursors. Stage 2 EBs (BW+VF) were cultured for 3 days in the presence of different induction factors. BW indicates BMP4 and Wnt3a; NC, no cytokine; V, VEGF; F, bFGF; L, LE540. (A) Hematopoietic potential of day 7 EB cells under different conditions were assessed by methylcellulose hematopoietic colony assay. Error bars indicate mean ± SEM (n = 4). Each sample was in triplicate. (B) Day 7 EB cells generated under different conditions were cultured as aggregates for 7 days with serum-free expansion medium supplemented with 10% FBS, SCF, FLT-3L, IL-3, and IL-6. Expression of CD45 was determined by flow cytometric analysis. Errors bars indicate mean ± SEM (n = 4). (C) Gene expression analysis by real-time PCR of day 7 EB cells under different conditions. The relative gene expression was normalized to the cells in culture without any induction factors in stage 3. Similar results were produced by 3 independent experiments with 3 parallel samples. The data were from one experiment, and data represent mean ± SEM.
To enhance the generation of multipotent hematopoietic progenitors, we tested the effect of RA at this stage.19-21 When RA was added to the stage 3 differentiation cultures (BW+VF+RA), the number of multilineage and myeloid colonies generated from day 7 EB cells increased dramatically (Figure 4A). Moreover, the number of erythroid lineage colonies was lower compared with cells under the spontaneous differentiation condition (Figure 4A). The 3 genes important for definitive hematopoiesis, Cmyb, GATA2, and HOXB4, were up-regulated in the presence of RA, whereas the erythroid genes GYPA, SCL, LMO2, and GATA1 were down-regulated (Figure 4C). Another definitive hematopoietic gene, RUNX1, was expressed at similar levels in both conditions (Figure 4C). These results show that RA enhances the generation of multipotent hematopoietic progenitors but decreases the production of erythroid progenitors.
To further assess the hematopoietic potential of cells under different conditions, we dissociated and then plated them to low-attachment plates in serum-free expansion medium supplemented with 10% FBS, SCF, Flt-3L, IL-3, and IL-6. After an additional 7 days of suspension culture, the hematopoietic commitment was assessed by the expression of CD45. In this assay, RA-treated cells (BW+VF+RA) generated the highest proportion (> 45%) of hematopoietic cells (Figure 4B). Approximately 20% of the cells without RA treatment in stage 3 (BW+VF+NC) were CD45+, and a similar percentage of the cells receiving LE540 treatment (BW+VF+L) in stage 3 expressed CD45 (Figure 4B). The up-regulation of the endothelial genes KDR, VE-Cad, and TIE2 in the presence of LE540 may reflect the proliferation of endothelial cells in the absence of RA signaling (Figure 4C), which is consistent with findings from studies in the mouse yolk sac.19,30 Approximately 10% of early stage 2 hemato-vascular precursor (BW+VF) cells also became CD45+ cells (Figure 4B), which suggests that these cells already have some hematopoietic potentials. Cells without VEGF/bFGF treatment at stage 2 (BW+NC+RA) failed to generate CD45+ cells. These cells also did not display the capacity to form hematopoietic colonies and completely failed to express hematopoietic genes (Figure 4A,C). The high-level expression of MEOX1 suggested that these cells contained paraxial mesoderm cells (Figure 4C). This finding is consistent with our previous result that RA induces paraxial mesoderm from immature mesoderm cells and further confirms the critical roles of VEGF and bFGF in hemato-vascular lineage specification.
Taken together, these results show that adding RA in stage 3 enhances the generation of hematopoietic progenitors, in contrast to the robust development of erythroid progenitors in spontaneous differentiation culture. Inhibition of endogenous RA signaling at this stage may allow the proliferation of endothelial cells.
Enrichment and characterization of hESC-derived hematopoietic progenitors
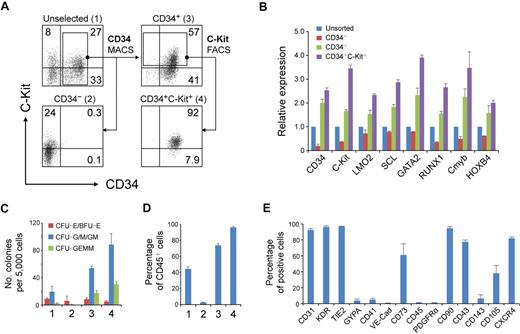
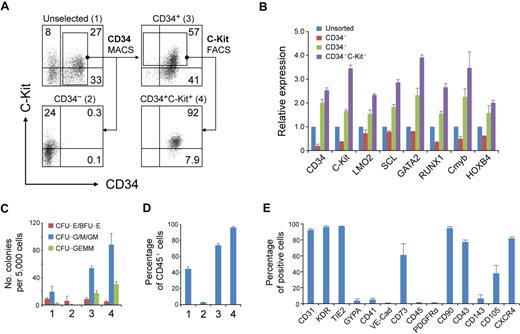
To enrich RA-induced hematopoietic progenitors, we separated day 7 cells according to the different expression patterns of CD34 and C-Kit with the use of magnetic cell sorting and fluorescence-activated cell sorting (Figure 5A). As expected, the CD34+ (population 3) cells expressed higher levels of hematopoietic genes than did the unselected (population 1) cells (Figure 5B). Separation of CD34+C-Kit+ (population 4) cells further increased the expression of these genes (Figure 5B). Furthermore, the CD34+C-Kit+ cell population generated the highest number of hematopoietic colonies (Figure 5C). After 7 days of differentiation, almost all of these cells expressed CD45, compared with approximately 75% of CD34+ cells and less than 10% of CD34− cells (Figure 5D). These results suggest that the CD34+C-Kit+ population is highly enriched for hematopoietic progenitors in day 7 RA-induced cells.
Hematopoietic progenitors enriched in CD34+ C-Kit+ population. (A) Magnetic cell sorting (MACS) and fluorescence-activated cell sorting (FACS) selection of day 7 EB cells by CD34 and C-Kit. The expression of CD34 and C-Kit in unselected cells (1), in MACS-selected CD34− cells (2), in MACS-selected CD34+ cells (3), and in FACS-selected CD34+C-Kit+ cells (4) was examined by flow cytometric analysis. Numbers represent percentage of total cells. (B) Gene expression of selected and unselected cells was assessed by real-time PCR. Relative gene expression was determined by normalization to that in unsorted cells. Similar results were produced by 3 independent experiments with 3 parallel samples. The data were from one experiment, and data represent mean ± SEM (C) Hematopoietic progenitor potential of MACS-selected and unselected cells were tested by methylcellulose hematopoietic colony assay. Error bars indicate mean ± SEM (n = 3). Each sample was in triplicate. (D) MACS-selected cells were cultured as aggregates for 7 days with serum-free expansion medium supplemented with 10% FBS, SCF, Flt-3L, IL-3, and IL-6. The hematopoietic potential of selected and unselected cells was assessed by CD45 expression determined by flow cytometric analysis. Error bars indicate mean ± SEM (n = 3). (E) Quantification of the percentage of CD34+ C-Kit+ hESC-derived hematopoietic progenitors expressing a given marker by flow cytometry.
Hematopoietic progenitors enriched in CD34+ C-Kit+ population. (A) Magnetic cell sorting (MACS) and fluorescence-activated cell sorting (FACS) selection of day 7 EB cells by CD34 and C-Kit. The expression of CD34 and C-Kit in unselected cells (1), in MACS-selected CD34− cells (2), in MACS-selected CD34+ cells (3), and in FACS-selected CD34+C-Kit+ cells (4) was examined by flow cytometric analysis. Numbers represent percentage of total cells. (B) Gene expression of selected and unselected cells was assessed by real-time PCR. Relative gene expression was determined by normalization to that in unsorted cells. Similar results were produced by 3 independent experiments with 3 parallel samples. The data were from one experiment, and data represent mean ± SEM (C) Hematopoietic progenitor potential of MACS-selected and unselected cells were tested by methylcellulose hematopoietic colony assay. Error bars indicate mean ± SEM (n = 3). Each sample was in triplicate. (D) MACS-selected cells were cultured as aggregates for 7 days with serum-free expansion medium supplemented with 10% FBS, SCF, Flt-3L, IL-3, and IL-6. The hematopoietic potential of selected and unselected cells was assessed by CD45 expression determined by flow cytometric analysis. Error bars indicate mean ± SEM (n = 3). (E) Quantification of the percentage of CD34+ C-Kit+ hESC-derived hematopoietic progenitors expressing a given marker by flow cytometry.
To further characterize these CD34+C-Kit+ hematopoietic progenitors, we examined the expression of other markers by flow cytometry (Figure 5E). Most CD34+C-Kit+ cells expressed CD31, KDR, and TIE2, reflecting the common origin of hematopoietic and endothelial cells. However, another hemogenic endothelium and endothelial marker, VE-Cad, was only expressed in a few cells. We also found low levels of GYPA and CD41, 2 surface markers that are expressed in erythroid and megakaryocyte bipotent progenitors in differentiated hESCs.24,37 A recent study shows the mesenchymal potential of hESC-derived CD34+ cells from coculture differentiation system.38 CD73, which is broadly expressed in mesenchymal stromal cells, endothelial cells, and epithelial cells, was expressed by greater than 60% of our selected cells. However, considering that no PDGFRα expression was detected, the expression of CD73 may reflect the character of hESC derivatives but not indicate a mesenchymal origin of these cells.
Further analysis showed that most of the cells did not express CD45 but did express CD43 and CD90. Two markers used to identify hematopoietic lineages in hESCs or mouse ESCs, CD10539 and CD143,34 were expressed in a distinctive pattern in the CD34+ C-Kit+ cells. A marker that is important for the homing of hematopoietic cells, CXCR4, was expressed on approximately 85% of the cells.
Sensitivity to drug toxicity of hESC-derived hematopoietic progenitors is similar to that of cord blood CD34+ cells
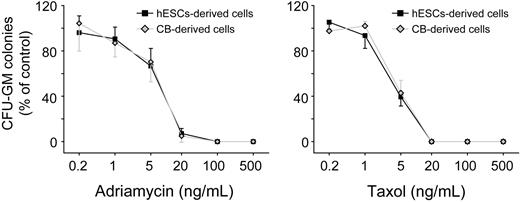
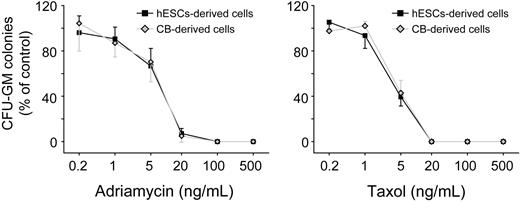
Human cord blood and bone marrow hematopoietic progenitors are used in vitro to assess myelotoxicity in preclinical drug development and to predict the acute neutropenia induced by chemotherapeutic drugs.40,41 To compare the sensitivity to drug toxicity of hESC-derived hematopoietic progenitors to cord blood cells, we plated both hESC-derived CD34+C-Kit+ cells and cord blood CD34+ cells into β-mercaptoethanol/erythropoietin-free methylcellulose. A series of doses of 2 well-known myelotoxic chemicals, doxorubicin and paclitaxel, were added to the culture. After 14 days in culture, hESC-derived hematopoietic progenitors and cord blood cells showed a similar pattern of percentage decreases in CFU-G/M/GMs in both drug groups (Figure 6D). This similarity in drug toxicity sensitivity suggests that hESC-derived hematopoietic progenitor cells could provide an alternative source of cells for toxicity testing in preclinical drug development. In these experiments, we used H1 hESCs. To test whether our induction method is generally applicable, we also conducted differentiation of H9 cells. As shown in supplemental Figure 8, similar hematopoietic differentiation efficiency was observed with the use of H9 cells.
Concentration-dependent inhibition of CFU-G/M/GM formation on exposure of hematopoietic progenitors to toxic chemicals. hESC-derived cells, hESCs-derived CD34+C-Kit+ hematopoietic progenitors; cord blood-derived cells, cord blood-derived CD34+ hematopoietic progenitors. Error bars indicate mean ± SEM (n = 3). Each sample was in triplicate. The relative colony numbers were normalized to DMSO-treated control samples. Adriamycin represents doxorubicin; and Taxal, paclitaxel.
Concentration-dependent inhibition of CFU-G/M/GM formation on exposure of hematopoietic progenitors to toxic chemicals. hESC-derived cells, hESCs-derived CD34+C-Kit+ hematopoietic progenitors; cord blood-derived cells, cord blood-derived CD34+ hematopoietic progenitors. Error bars indicate mean ± SEM (n = 3). Each sample was in triplicate. The relative colony numbers were normalized to DMSO-treated control samples. Adriamycin represents doxorubicin; and Taxal, paclitaxel.
Discussion
In this study, we established a 3-stage method directing the differentiation of hESCs toward hematopoietic progenitors. After 7 days of induction, greater than 25% of differentiated hESCs expressed CD34 and C-Kit (Figure 5A). These CD34+C-Kit+ hematopoietic progenitors expressed high levels of definitive hematopoietic genes RUNX1, GATA2, Cmyb, and HOXB4, and they gave rise to more than 95% of CD45+ hematopoietic cells (Figure 5B,D). Most of the CD34+C-Kit+ hematopoietic progenitors we obtained expressed CD43 and CD90 (Figure 5E), these characteristics are similar to those of hematopoietic progenitors obtained from the OP9 coculture protocol.24 However, a complete lack of CD45 expression suggested that our cells are at an early stage, which will facilitate studies on the early onset of human hematopoietic cells before the appearance of CD45. However, the low percentage of VE-Cad+ cells indicates that these cells differ from CD45negPFV hematopoietic precursors from human EBs,13 In addition, this suggests that our CD34+C-Kit+ hematopoietic progenitors are beyond the hemogenic endothelium stage. The hESC-derived hematopoietic progenitors also showed drug toxicity-sensitivity similar to that of cord blood CD34+ cells, which provides an alternative method for predicting chemically induced myelosuppression in preclinical drug development.40,41
During the second stage of hematopoietic differentiation, we found that endogenous RA signaling was required for the establishment of the hemato-vascular lineage induced by VEGF and bFGF, whereas exogenous addition of RA efficiently induced paraxial mesoderm (Figure 3). Because no early hematopoiesis defect has been reported in either Raldh2- or Rar-null mutant mice (reviewed in Niederreither and Dolle42 ), these findings suggest RA signaling is not required for early hemato-vascular development in mice. The role of RA signaling in the development of hemato-vascular precursor observed here may thus be due to the different roles of RA signaling in humans and mice. Moreover, because RA regulates HOX gene expression along the anterior-posterior axis (reviewed in Duester43 ), the abnormal patterning of mesoderm when RA signaling is inhibited by LE540 is also a possible explanation of this effect. In addition, the formation of paraxial mesoderm does not require RA signaling in mouse embryos, because the first RA activity occurs at embryonic day (E) 7.5,44 when paraxial mesoderm has already formed. Because the concentration of RA applied in vitro was much higher than the endogenous RA signal in mouse embryos (2μM vs 25nM45 ), induction of paraxial mesoderm by RA may be an overdose effect. Besides, in our differentiated EBs, RALDH2 was expressed at day 1.5, which is earlier than the appearance of all hematopoietic genes. However, in mouse embryos, Raldh2 appears at E7.5,46 when the first hematopoietic cells emerge. This discrepancy in the time at which RA activity starts may reflect the differences between hESC differentiation and embryo development or differences in developmental regulation between humans and mice, which also may explain the role of RA in early mesoderm specification observed here.
The effect of RA on hematopoietic progenitors we observed here depends on the addition of VEGF and FGF at stage 2. Without these 2 factors, the endogenous RA is not able to generate hematopoietic progenitors (Figure 4). Meanwhile, blocking endogenous of RA in the presence of VEGF and FGF also attenuates hematopoietic development (Figure 3). These results suggested a connection between RA and VEGF/FGF signaling. The relation between RA and FGF signaling during development has been widely documented (reviewed in Niederreither and Dolle42 and Duester43 ). A recent study that used mouse ESCs shows that RA orchestrates extracellular signal-related kinase/Fgf signaling, which is important for ESC differentiation.47 The more-detailed mechanisms of the connection between RA and VEFG/FGF signaling needs further studies.
In the third stage, we found that RA enhanced the generation of hematopoietic progenitors from hemato-vascular precursors, while suppressing erythroid development. Because the addition of LE540 at stage 3 did not inhibit the emergence of hematopoietic cells, the effect of RA may largely be due to an expansion role to hematopoietic progenitors, which is compatible with the roles of RA on hematopoietic stem/progenitor cells reported by other studies (reviewed in Collins48 and Evans49 ). The effect of RA at the third stage may act through the up-regulation of a panel of transcription factors important for development and proliferation of hematopoietic progenitors, HOXB4, GATA2, and Cmyb, and down-regulation of the erythropoiesis genes GATA1, SCL, and LMO2. Because RA regulates the expression of the HOX gene cluster during early organogenesis in mice,42,43 the up-regulation of HOXB4 in hematopoietic development was not surprising. In chicks, RA is important for yolk sac hematopoiesis and may regulate GATA2 expression through BMP signals.18 In Xenopus, RA greatly inhibits the expression of GATA1 during late blastula and early gastrula stages, but it does not affect GATA2.20 Other work shows that, in Xenopus embryos, activating RA receptors up-regulate GATA2 expression and down-regulate LMO2 through the neurula stage.50 Direct interaction between Rarα and Gata2 has also been shown.51 Thus, the up-regulation of GATA2, as well as the down-regulation of GATA1, LMO2, and SCL, was expected, because these 3 factors form a complex and regulate erythropoiesis synergistically.52,53 The direct effect of RA signals on Cmyb during hematopoietic development has not been reported. The activation of Cmyb expression observed here may occur through other signaling pathways downstream of RA.
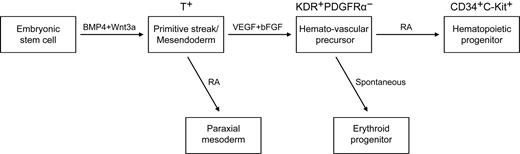
The effects of RA signaling on the generation of hematopoietic cells vary widely among species.18,19,21 An early study in Xenopus embryos shows that RA treatment causes a large decline in the expression of α-globin and in the number of red blood cells.21 A study that used vitamin A–deficient quail embryos shows that retinoids are required for yolk sac hematopoiesis.18 However, in Raldh2−/− mice, no hematopoiesis deficiency occurs in the yolk sac before E8.0.19 That study also showed that at E9.5, Raldh2−/− yolk sac single-positive (SP) cells generate only erythroid colonies and fail to develop other types of colonies. Moreover, the emergence of hemogenic endothelium (Flk1+CD45− SP) and multipotent (Flk1− CD45+ SP) hematopoietic progenitors in Raldh2−/− yolk sac decreases dramatically, and the remaining hemogenic endothelium and hematopoietic progenitors fail to generate any hematopoietic colonies.19 These data suggest that RA signaling regulates the generation of hematopoietic cells. On the basis of the previous studies and our current work, we propose a stage-specific role of RA in human mesoderm and hematopoietic development (Figure 7). The exogenous addition of RA efficiently induced paraxial mesoderm at mesoderm specification stage. At a later stage, RA enhanced the generation of hematopoietic progenitors, while suppressing erythropoiesis. During these process, stage 2 EB cells (day4) generated a large number of blast colonies, but only little hematopoietic colonies formed (Figures 1,3–4). After further differentiation, the number of CFCs increases dramatically. These findings suggested that the KDR+PDGFR− hemato-vascular precursors and the CD34+C-Kit+ hematopoietic progenitors develop sequentially.
A model of the stage-specific role of RA in human mesoderm and hematopoietic development. VEGF and bFGF efficiently specify KDR+PDGFRα− the hemato-vascular lineage from BMP4/Wnt3a-induced T+ primitive streak/mesendoderm cells. At this stage, the role of exogenous RA is to induce paraxial mesoderm. At a later stage, RA enhances the generation of hematopoietic progenitors (CD34+C-Kit+) and decreases erythroid development from hemato-vascular precursors.
A model of the stage-specific role of RA in human mesoderm and hematopoietic development. VEGF and bFGF efficiently specify KDR+PDGFRα− the hemato-vascular lineage from BMP4/Wnt3a-induced T+ primitive streak/mesendoderm cells. At this stage, the role of exogenous RA is to induce paraxial mesoderm. At a later stage, RA enhances the generation of hematopoietic progenitors (CD34+C-Kit+) and decreases erythroid development from hemato-vascular precursors.
In summary, we established a chemically defined 3-stage strategy to generate hematopoietic cells from hESCs and proposed a stage-specific role for RA in human mesoderm and hematopoietic development. These results will facilitate future studies of the molecular mechanisms that regulate hematopoietic development and may provide prospects for obtaining an alternative source for hematopoietic cell transplantation in humans. Preliminary transplantation experiments have shown that the engraftment ability of our CD34+C-Kit+ cells was quite low. A recent study showed the critical role of the AGM niche in the generation of hematopoietic stem cells.54 However, specific signaling pathways remain to be identified in controlling this process. We hope that signals from the AGM region could combine with our system in the future to generate robust engraftable hematopoietic cells from hESCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Yizhe Zhang for providing technical support in real-time PCR and Liying Du in FACS analysis. We also thank Chengyan Wang, Hongxia Lu, Pengbo Zhang, Xiaomeng Sun, Yan Shi, Yang Zhao, Donghui Zhang, Wei Jiang, Dongxin Zhao, Qiang Zhang, and other colleagues in our laboratory for proving technical assistance and discussion in the preparation of this manuscript.
This work was supported by the Bill & Melinda Gates Foundation (grant 37871), National Science and Technology Major Project (grants 2008ZX10001-010 and 2009ZX10004-403), the National Basic Research Program of China (973 program, grants 2005CB522903, 2006CB504203, 2007CB947901, 2010CB945200, and 2011CB964800), National Natural Science Foundation of China for Creative Research Groups (grant 30421004), and the 111 project (H.D.).
Authorship
Contribution: C.Y. designed and performed research, analyzed data, and wrote the paper; Y. Liu designed and performed research and analyzed data; Z.M. performed research; M.Y. designed research and provided administrative support; W.L. performed research; Y. Lv performed research; M.D. designed research and wrote the paper; and H.D. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hongkui Deng, Department of Cell Biology, College of Life Sciences, Peking University, Beijing, China, 100871; e-mail: hongkui_deng@pku.edu.cn.