Abstract
Even the most potent immunosuppressive drugs often fail to control graft-versus-host disease (GVHD), the most frequent and deleterious posttransplantation complication. We previously reported that photodepletion using dibromorhodamine (TH9402) eliminates T cells from healthy donors activated against major histocompatibility complex–incompatible cells and spares resting T cells. In the present study, we identified photodepletion conditions selectively eradicating endogenous proliferating T cells from chronic GVHD patients, with the concomittant sparing and expansion of CD4+CD25+ forkhead box protein 3–positive T cells. The regulatory T-cell (Treg) nature and function of these photodepletion-resistant cells was demonstrated in coculture and depletion/repletion experiments. The mechanism by which Tregs escape photodepletion involves active P-glycoprotein–mediated drug efflux. This Treg-inhibitory activity is attributable to interleukin-10 secretion, requires cell-cell contact, and implies binding with cytotoxic T-lymphocyte antigen 4 (CTLA-4). Preventing CTLA-4 ligation abrogated the in vitro generation of Tregs, thus identifying CTLA-4–mediated cell-cell contact as a crucial priming event for Treg function. Moreover, the frequency of circulating Tregs increased in chronic GVHD patients treated with TH9402 photodepleted cells. In conclusion, these results identify a novel approach to both preserve and expand Tregs while selectively eliminating CD4+ effector T cells. They also uncover effector pathways that could be used advantageously for the treatment of patients with refractory GVHD.
Introduction
Patients with chronic graft-versus-host disease (GVHD) suffer from considerable organ damage, leading to significant morbidity, frequent hospitalizations, and high mortality rates.1-3 While potent immunosuppressive agents have been developed, a large number of patients either fail to respond or develop secondary treatment refractoriness.4-6 Moreover, their prevalent side effects emphasize the need for innovative treatment strategies. Cellular therapy in the form of extracorporeal photopheresis (ECP) offers an interesting therapeutic option in terms of both efficacy and safety. It mostly utilizes 8-methoxypsoralen as a photosensitizer and ultraviolet illumination to provoke immune modulation.7-9
The new-generation photosensitizer, 4,5-dibromorhodamine-123 (TH9402), is an attractive candidate for ECP because it demonstrates particularly potent cytotoxicity, uses visible light, and its unique ability to preferentially eliminate activated over resting T cells may translate into an appealing approach to eliminate pathogenic host-reactive T cells in patients with chronic GVHD.10 Indeed, T cells activated upon exposure to mitogens or major histocompatibility complex (MHC)–disparate targets in a mixed lymphocyte reaction (MLR) are eliminated after TH9402 photodepletion.11,12 Such photodepletion of alloreactive cells also prevents the development of GVHD after MHC-mismatched transplantation and preserves graft-versus-leukemia activity in a murine model.13 Moreover, a phase I clinical trial of allogeneic MHC-mismatched transplantation, followed by infusion of donor lymphocytes depleted of their alloreactive component using TH9402 photodepletion, also provides evidence that such photodepletion can prevent the development of infections without causing GVHD.14 Interestingly, some CD4+CD25+ cells are resistant to TH9402 photodepletion,11 and the fact that this cell subset is forkhead box protein 3 positive (FOXP3+) further suggests that these cells might be regulatory T cells (Tregs), and could be used for the treatment of GVHD.9,15-19 However, these findings were obtained after the photodepletion of healthy donor cells activated in vitro, rather than cells from GVHD patients who demonstrate immune dysregulation. In addition, whether Tregs surviving photodepletion have preserved function, and how they exert their activity, has not been investigated.
The aim of the present study was to determine whether TH9402 photodepletion could be used to generate Tregs while selectively eliminating endogenous host-reactive T cells circulating in the peripheral blood of patients with chronic GVHD. We first identified novel photodepletion conditions inhibiting T-cell proliferation through the concomitant depletion of host-reactive T cells and the preservation of functional Tregs. In addition, the ability of photodepleted GVHD cells to abrogate the proliferation of untreated patient GVHD cells was mediated by CD4+CD25+FOXP3+ cells, as demonstrated in depletion/repletion studies. Moreover, we found that the inhibitory function of photodepletion-resistant Tregs was attributable to interleukin-10 (IL-10) secretion, and that their inhibitory activity required initial exposure of these photodepleted cells to untreated cells from chronic GVHD patients. Finally, cell-cell contact through cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) was crucial to the suppressive effect of Tregs as well as to their production, which was observed both in vitro and in vivo. Thus, with its dual effects on Tregs and host-reactive cells, TH9402 photodepletion represents a particularly appealing alternative for the treatment of patients with steroid-refractory chronic GVHD.
Methods
Patients and healthy donors
Peripheral blood samples were obtained from healthy donors (n = 5) and from chronic GVHD patients (n = 10) failing to respond to at least 2 different immunosuppressive agents, including corticosteroids. These patients all underwent allogeneic stem cell transplantation at Hopital Maisonneuve-Rosemont (HMR) and suffered from extensive chronic GVHD, according to the National Institutes of Health (NIH) Consensus Development Project criteria.20 Additional samples from 5 patients transplanted at HMR (n = 3), Vancouver General Hospital (n = 1), and Ottawa Hospital (n = 1), and satisfying these criteria, were evaluated at weeks 0, 2, 4, and 8 of weekly ECP treatments in a phase 1/2 clinical trial evaluating the safety as well as biologic and clinical efficacy of ECP using TH9402-treated apheresis products in subjects with extensive steroid-refractory chronic GVHD. Laboratory studies were approved by the HMR Ethics Committee, and written informed consent was obtained from all patients and healthy donors in accordance with the Declaration of Helsinki. The clinical photodepletion protocol was approved by each institutional ethics committee.
Photodepletion
Peripheral blood mononuclear cells (PBMCs) from chronic GVHD patients were isolated using density-gradient centrifugation (Ficoll-Hypaque; Amersham Pharmacia Biotech) and resuspended at a concentration of 1 × 106 cells/mL in X-Vivo 15 medium without phenol red (BioWhittaker), supplemented with 2.5% heat-inactivated fetal bovine serum (iFBS; Hyclone). Cells were incubated for 45 minutes in the presence of 1.32μM 4,5-dibromorhodamine (TH9402; Kiadis Pharma) at 37°C, in 5% CO2, and exposed in T75 flasks (VWR) to 5 J/cm2 visible light (514 nm) delivered by a scanning device (Kiadis Pharma). To determine the role of P-glycoprotein in Treg photodepletion, cells were exposed to 50μM verapamil (Sigma-Aldrich) during the incubation with TH9402 and light exposure. Then, viability, phenotypic analysis, and Treg function were evaluated.
Patients treated under the clinical phase 1/2 study of ECP underwent 7 weekly leukapheresis followed by photodepletion ex vivo using the above-described TH9402 treatment protocol, except that iFBS was replaced by autologous serum, and 1 × 108 photodepleted cells were reinfused to the patient on the same day.
Regulatory T-cell function
TH9402 photodepletion or untreated cells corresponded to effector (E) cells, while untreated cells from the same GVHD patient were used as targets (T). To measure Treg function, effector cells were incubated with target cells at E:T ratios ranging from 1:16 to 1:1 in round bottomed, 96-well or Transwell plates (both from Corning) at 37°C under 5% CO2 for 5 days. Culture medium consisted of RPMI 1640 supplemented with 10% iFBS, 1mM sodium pyruvate, modified Eagle medium nonessential amino acids, 2μM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Gibco), 5 × 10−5M β-mercaptoethanol (Sigma-Aldrich), and 50 IU/mL IL-2 (R&D Systems). Cell proliferation was measured after 5-day cultures by pulsing cells with 1 μCu/well (0.037 mBq) 3H-thymidine (PerkinElmer Life and Analytical Sciences) for the last 18 hours. Cells were then harvested using a Packard Filtermate Cell Harvester-Unifilter 96 unit (Packard) onto Unifilter plates (Millipore), and 3H-thymidine incorporation was measured using a Topcount NXT Microplate Scintillation and Luminescence counter (Packard). Similarly, healthy donor control PBMCs were stimulated by exposure for 96 hours at 37°C under 5% CO2 to MHC-mismatched cells irradiated (Gammacell 3000 Elan; MDS Nordion) at a dose of 6000 cGy as previously described.11 Treg activity was measured after cells were harvested and exposed (or not) to photodepletion.
EdU proliferation assay
Cell proliferation was also measured after the addition of 10μM EdU (5-ethynyl-2′-deoxyuridine; Invitrogen) to the culture. EdU incorporation was evaluated using Pacific Blue–conjugated azide (Invitrogen) and analyzed by flow cytometry (FACS LRS II; BD Biosciences). Lectin-mediated T-cell activation was analyzed after 72-hour incubation with 2 μg of phytohemagglutinin A (PHA; Sigma-Aldrich).
Flow cytometric analysis
Flow cytometric assessment was performed using the following: fluorescein isothiocyanate (FITC)–conjugated anti-CD4 (clone RPA-T4), anti-CD8 (clone RPA-T8), anti-CD25 (clone M-A251), phycoerythrin (PE)–conjugated anti-CD25 (clone M-A251), allophycocyanin (APC)– conjugated anti-CD8 (clone RPA-T8; all from BD Biosciences), and peridinin chlorophyll-protein complex (PerCP)–conjugated anti-CD4 (clone MEM-241; Abcam) monoclonal antibodies (mAbs). For intracellular staining, PE-conjugated anti-FOXP3 (clone PCH101; eBioscience) was used according to the manufacturer's instructions. Intracellular IL-10 expres-sion was measured using an APC-conjugated anti–human IL-10 antibody (clone JES3–19F1; Biolegend). Before staining, cytokine production was stimulated by 0.05 μg/mL PMA/0.5 μg/mL ionomycin for 4 hours and 50 μg/mL brefeldin A (all from Sigma-Aldrich) added for the last 2 hours. All stainings were made in the presence of unconjugated FcR blocking reagent (Miltenyi Biotec) at saturation to prevent nonspecific staining. In addition, 7-amino-actinomycin D (7-AAD; BD Biosciences) was added before analysis of cells without intracellular staining to exclude dead cells. CD4+CD25− cells were isolated and stained with a mitochondrial dye (Mitotracker Deep Red; Invitrogen) before coculture to enable their discrimination from the CD4+CD25+ cells added. FOXP3 analysis of cells from patients in the clinical study was performed in triplicate. At least 5 × 104 events were acquired using FACS LSRII (BD Biosciences) and analyzed using FlowJo software Version 7.5.5 (TreeStar).
Regulatory T-cell depletion and isolation
Tregs were depleted or reintroduced before photodepletion using the CD4+CD25+ human regulatory T-cell isolation kit (Miltenyi Biotec), which consisted of a negative selection for CD4+ cells, followed by a positive selection for CD25+ cells using magnetic microbeads conjugated antibodies and the MACS LS columns (Miltenyi Biotec). After isolation of CD4+CD25+ cells, Tregs were added back to Treg-depleted (CD4+CD25−) PBMCs. Repletion conditions implied the reintroduction of Tregs (CD4+CD25+) before photodepletion and in proportions identical to those of the initial cell product.
Cytokine titration
Photodepletion-treated and -untreated cells from chronic GVHD patients were cocultured with untreated cells from the same patient at different E:T cell ratios for 96 hours. Cells were then harvested, and supernatants were collected and stored at −80°C until analysis. Cytokine titration was performed using enzyme-linked immunosorbent assay (ELISA) kits for interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-10 (Assay Design), and transforming growth factor-β (TGF-β; Biosource), following manufacturers' protocols.
Functional inhibition of Treg mediators
Biologic activity of Treg mediators was inhibited using anti–IL-10, anti–TGF-β, anti-GITR (glucocorticoid-induced tumor necrosis factor receptor; R&D Systems), anti–CTLA-4 (Exalpha Biological), or isotype-matched irrelevant mouse mAbs (Sigma-Aldrich). To ensure inhibition by the mAbs, we used concentrations 10 times greater than the 50% neutralizing dose (ND50) provided by the manufacturer. Cells were then cocultured for 5 days, and proliferation was determined by measuring 3H-thymidine uptake.
Statistical analysis
Photodepletion and untreated cell numbers were compared using the Mann-Whitney test. GVHD cell proliferations in the presence of photodepletion or untreated cells were compared using analysis of variance (ANOVA) tests. Statistical analyses were performed using GraphPad Prism Version 4.00 for Windows (GraphPad Software).
Results
Photodynamic inhibition of proliferating cells from GVHD patients
To harness the therapeutic potential of TH9402 photodepletion for patients with immune dysregulation, we evaluated several photodepletion processing parameters (eg, concentration, incubation time, dye efflux period, and light energy). A first evaluation using healthy control cells allowed us to identify treatment conditions by eliminating greater than 75% of MHC-mismatched MLR-activated cells (data not shown). The capacity of this novel photodepletion approach, incorporating a low (1.32μM) TH9402 concentration, to eliminate endogenously proliferating T cells was then evaluated in patients with chronic GVHD. Because chronic GVHD has been shown to be predominantly due to noncytotoxic host-reactive helper T lymphocytes directed against peptides on MHC class II molecules, our studies focused on the elimination of this cell population.21-23
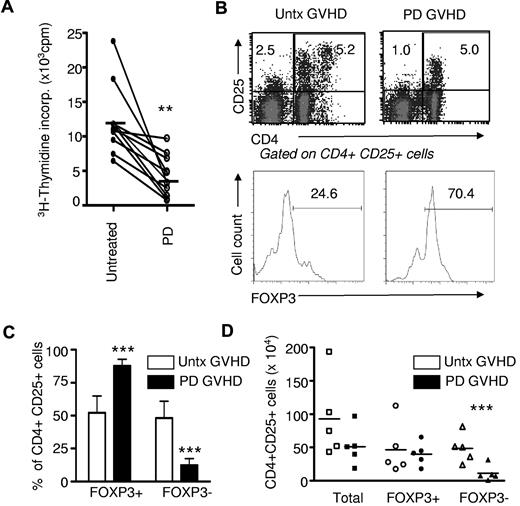
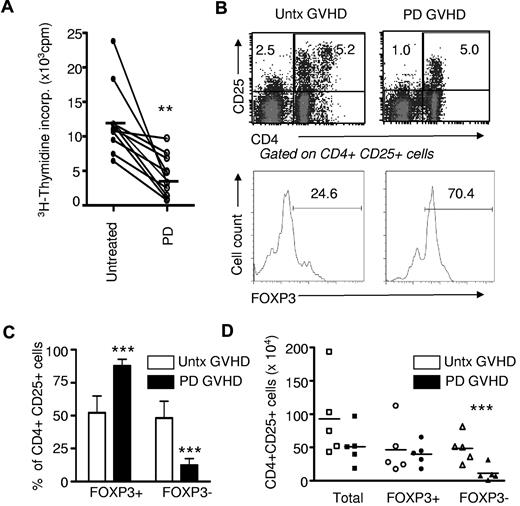
All GVHD patients tested (n = 10) had severe extensive chronic disease and were refractory to at least 2 immunosuppressive agents. Photodepletion under these novel treatment conditions induced a dramatic decrease in GVHD patient-cell proliferation, as measured in a conventional 3H-thymidine incorporation assay (10 906 ± 5177 and 4226 ± 3056, mean ± SD, no photodepletion and photodepletion, respectively; n = 10; P < .001; Figure 1A). The decreased 3H-thymidine incorporation in IL-2–supplemented culture medium could result from decreased proliferation or increased apoptosis of PBMCs from GVHD patients.
Photodepletion (PD) cells display reduced proliferation and mostly a Treg phenotype. (A) PBMCs from chronic GVHD patients (n = 10) were exposed (or not) to photodepletion. Proliferation of GVHD cells was assessed by 3H-thymidine incorporation after 5-day cultures in the presence of IL-2. (B) Three days after photodepletion, CD4+CD25+ cells were evaluated by flow cytometry and activated, and Treg cells were discriminated by FOXP3 expression. Shown are representative examples and a compilation of 6 independent experiments ± SD expressed in relative (C) and absolute (D) number. Clear bars and filled bars represent untreated (untx) and photodepletion-treated GVHD PBMCs, respectively. **P < .01 and ***P < .001
Photodepletion (PD) cells display reduced proliferation and mostly a Treg phenotype. (A) PBMCs from chronic GVHD patients (n = 10) were exposed (or not) to photodepletion. Proliferation of GVHD cells was assessed by 3H-thymidine incorporation after 5-day cultures in the presence of IL-2. (B) Three days after photodepletion, CD4+CD25+ cells were evaluated by flow cytometry and activated, and Treg cells were discriminated by FOXP3 expression. Shown are representative examples and a compilation of 6 independent experiments ± SD expressed in relative (C) and absolute (D) number. Clear bars and filled bars represent untreated (untx) and photodepletion-treated GVHD PBMCs, respectively. **P < .01 and ***P < .001
These results prompted us to evaluate the impact of photodepletion on CD4+CD25+ cells, which should correspond to proliferating cells after culture for 3 days in the presence of IL-2. Interestingly, a substantial number of these cells escaped photodepletion (Figure 1B-D). However, 2 types of CD4 T cells express CD25 and therefore proliferate extensively in the presence of IL-2: “activated” T cells, including host-reactive CD4 T cells with a pro-GVHD activity (CD4+CD25+FOXP3−), and Treg (CD4+CD25+FOXP3+) cells. We therefore sought to evaluate the effect of TH9402 photodepletion on these 2 cell types. We found that TH9402 photodepletion did not affect the absolute number of CD4+CD25+FOXP3+ cells, but significantly decreased the number of CD4+CD25+FOXP3− (Figure 1B-D). As a result, photodepletion led to a major decrease in the proportion of effector-memory phenotype CD4+CD25+FOXP3− cells (P < .001).
Photodepletion preserves functional Tregs from healthy individuals
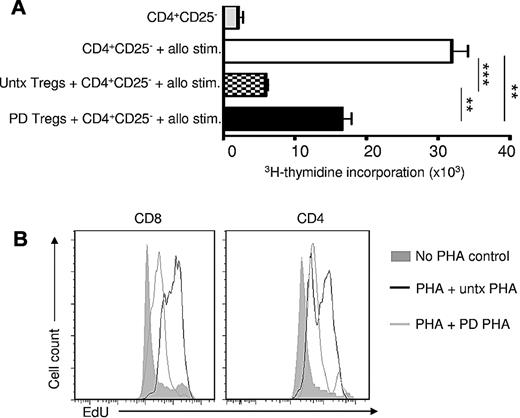
To gain further insight into the susceptibility of Tregs to photodepletion and to ensure that Tregs escaping photodepletion were functional, CD4+CD25+ cells (greater than 95% purity) were immunomagnetically isolated from healthy individuals. These CD4+CD25+ cells underwent photodepletion (or not), incubation with CD4+CD25− cells, and subsequent exposure to allogeneic stimulators for 5 days. CD4+CD25− cells demonstrated a solid proliferative response to allogeneic MHC-mismatched stimulators (Figure 2A). The addition of CD4+CD25+ Tregs, whether exposed or not to photodepletion, thwarted substantially the CD4 response (P < .01 and P < .001, respectively; n = 3). Photodepletion had a minimal effect on the function of Tregs (P < .01), but preserved most Treg activity.
Photodepletion (PD) preserves functional Tregs. (A) CD4+CD25− cells from healthy donors were cultured with allogeneic MHC-mismatched cells for 5 days either alone or in the presence of autologous untreated (untx) or photodepletion-treated CD4+CD25+ cells (Tregs) from autologous donors. Proliferation was evaluated by measuring 3H-thymidine incorporation (mean ± SEM; n = 5). (B) PHA-activated PBMCs from healthy donors were exposed (or not) to photodepletion and admixed with autologous PHA-activated PBMCs. Three days later, CD4 and CD8 cell proliferation was determined by flow cytometry using EdU incorporation. Plot shown is representative of 3 independent experiments **P < .01 and ***P < .001
Photodepletion (PD) preserves functional Tregs. (A) CD4+CD25− cells from healthy donors were cultured with allogeneic MHC-mismatched cells for 5 days either alone or in the presence of autologous untreated (untx) or photodepletion-treated CD4+CD25+ cells (Tregs) from autologous donors. Proliferation was evaluated by measuring 3H-thymidine incorporation (mean ± SEM; n = 5). (B) PHA-activated PBMCs from healthy donors were exposed (or not) to photodepletion and admixed with autologous PHA-activated PBMCs. Three days later, CD4 and CD8 cell proliferation was determined by flow cytometry using EdU incorporation. Plot shown is representative of 3 independent experiments **P < .01 and ***P < .001
To confirm the immunosuppressive effect of photodepletion cells and to determine whether their inhibitory effect would be sufficiently potent to abrogate a very strong proliferative signal, lectin (PHA)–activated PBMCs from healthy individuals underwent TH9402 photodepletion and subsequent coculture with untreated autologous PHA-primed cells. Cellular proliferation, as evaluated by EdU labeling after 3 days of coculture (Figure 2B), showed that the addition of photodepleted PBMCs decreased cell divisions in response to PHA from CD4+ and CD8+ cells. These results demonstrate that photodepletion cells' inhibitory activity is present against both CD4+ and CD8+ lymphocytes, and that this cell-mediated inhibition of proliferation is sufficiently robust to reduce cell proliferation in response to a very potent proliferative signal.
Treg enrichment and expansion after photodepletion of GVHD patient cells
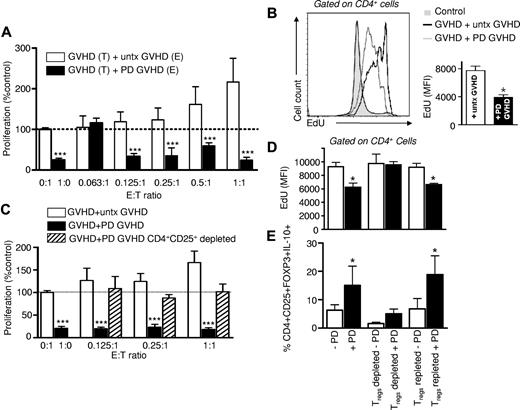
Because photodepletion spared Tregs from healthy individuals, and cells corresponding immunophenotypically to Tregs from GVHD patients, we determined the impact of photodepletion on Treg functionality of GVHD patient cells. When PBMCs from GVHD patients having undergone TH9402 photodepletion were incubated for 5 days with the same patient's untreated cells, they induced a drastic inhibition of untreated cell proliferation (Figure 3A; E:T 0.125:1 to 1:1 vs untreated targets alone [0:1] cells, P < .001; n = 6). By contrast, the addition of graded numbers of untreated GVHD effector cells to GVHD target cells led to enhanced global proliferation that correlated with the increase in untreated cell numbers. This ultimately resulted in a doubling of the initial target cell proliferation level upon the addition of an identical number of untreated effectors to the targets (1:1). In addition, increasing the effector-to-target ratios resulted in an enhancement of the differential proliferative activity of target cells exposed to treated versus untreated effectors (P < .001). Thus, photodepletion of GVHD patient cells inhibited spontaneous (E:T ratio of 1:0) prolifera-tion and suppressed autologous MLR reactivity. Interestingly, effector-cell incubation with TH9402 without light exposure, or with light exposure but without TH9402, had no effect on proliferation, while combining TH9402 and light caused a major inhibition of target cell proliferation (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), confirming the specificity of the photoactive treatment.
Photodepletion cells from GVHD patients demonstrate Treg inhibitory activity. (A) Effector (E) PBMCs from GVHD patients were exposed (filled bars) or not (clear bars) to photodepletion and cocultured at indicated ratios with target (T) PBMCs from the same patient for 5 days. Proliferation was evaluated by measuring 3H-thymidine incorporation. Bars represent mean ± SEM of 6 independent experiments. (B) Experiment similar to (A) at a 1:1 ratio, where specific proliferation of CD4+ cells was measured by incorporation of EdU. Shown are a representative and a compilation of 3 independent experiments (mean ± SD). (C) Untreated, photodepletion-exposed, or CD4+CD25+-depleted/photodepletion-exposed PBMCs from GVHD patients were cocultured at indicated ratios with autologous PBMCs for 5 days. Proliferation was evaluated by measuring 3H-thymidine incorporation (n = 3). (D-E) PBMCs from healthy donors were stimulated by MHC-incompatible PBMCs at a 1:1 ratio and exposed (filled) or not (clear bars) to photodepletion. Cells were either left untouched, depleted, or repleted in CD4+CD25+ cells and placed into coculture with equal numbers of MLR cells from the same donors for 5 days. (D) CD4+ cell proliferation was assessed by EdU incorporation (mean ± SEM), and (E) CD25+FOXP3+IL-10+ cells within the CD4+ cell population (mean ± SD) was estimated by flow cytometry. *P < .05 and ***P < .001
Photodepletion cells from GVHD patients demonstrate Treg inhibitory activity. (A) Effector (E) PBMCs from GVHD patients were exposed (filled bars) or not (clear bars) to photodepletion and cocultured at indicated ratios with target (T) PBMCs from the same patient for 5 days. Proliferation was evaluated by measuring 3H-thymidine incorporation. Bars represent mean ± SEM of 6 independent experiments. (B) Experiment similar to (A) at a 1:1 ratio, where specific proliferation of CD4+ cells was measured by incorporation of EdU. Shown are a representative and a compilation of 3 independent experiments (mean ± SD). (C) Untreated, photodepletion-exposed, or CD4+CD25+-depleted/photodepletion-exposed PBMCs from GVHD patients were cocultured at indicated ratios with autologous PBMCs for 5 days. Proliferation was evaluated by measuring 3H-thymidine incorporation (n = 3). (D-E) PBMCs from healthy donors were stimulated by MHC-incompatible PBMCs at a 1:1 ratio and exposed (filled) or not (clear bars) to photodepletion. Cells were either left untouched, depleted, or repleted in CD4+CD25+ cells and placed into coculture with equal numbers of MLR cells from the same donors for 5 days. (D) CD4+ cell proliferation was assessed by EdU incorporation (mean ± SEM), and (E) CD25+FOXP3+IL-10+ cells within the CD4+ cell population (mean ± SD) was estimated by flow cytometry. *P < .05 and ***P < .001
To further investigate the photodepletion-mediated inhibition of proliferation in nonphotodepleted cells, patient cells undergoing photodepletion were added to untreated cells at a 1:1 ratio, and their proliferation was measured using EdU flow cytometric labeling (Figure 3B). The incubation of photodepleted GVHD cells with untreated cells induced a net decrease in their DNA replication (ie, EdU incorporation) level in comparison with cells incubated with nonphotodepleted GVHD cells. However, the former showed higher proliferation than cells from healthy donors, demonstrating that GVHD cells having undergone photodepletion still had the intrinsic ability to replicate. To determine whether the inhibitory effect of photodepletion cells could be ascribed to CD4+CD25+ Tregs, CD4+CD25+ cells were immunomagnetically removed from photodepletion cells before their addition to target cells. Depletion of CD4+CD25+ cell resulted in the complete abrogation of photodepletion-mediated inhibition of target cell proliferation under conditions where the same number of non-CD4+CD25+ depleted photodepletion cells showed clear antiproliferative activity (P < .001; Figure 3C). Thus, these results provide solid evidence that the inhibitory effect of photodepletion cells is mediated by CD4+CD25+ Tregs.
Treg depletion/repletion regulates the photodepletion inhibitory effect
To further assess the role of Tregs in photodepletion-mediated inhibition of target cell proliferation, MLR-activated effector cells underwent photodepletion in the context of Treg depletion and repletion (Figure 3D-E). Photodepletion of MLR-activated cells decreased DNA replication upon their addition to untreated MLR targets at a 1:1 ratio (Figure 3D). In contrast, Treg depletion of effector cells before photodepletion increased target cell proliferation to the level of cells exposed to nonphotodepletion effector cells. Treg repletion restored the suppressive potential of photodepletion cells. Indeed, the proportion of IL-10–secreting Tregs more than doubled under photodepletion conditions, whether untreated or after Treg repletion (Figure 3E). Interestingly, in nonphotodepletion conditions, neither Treg depletion nor repletion alone affected cell proliferation, suggesting that Treg numbers have to surpass a defined threshold to exert a significant effect. These depletion/repletion experiments, thus confirmed the major role of Tregs in the photodepletion-mediated inhibition of proliferation.
The inhibitory effect is mediated by postphotodepletion Tregs
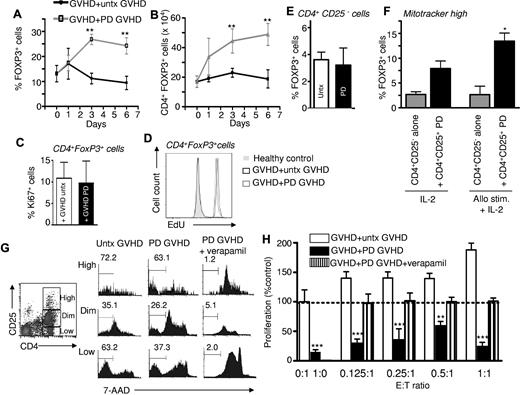
We then sought to evaluate more precisely the impact of photodepletion on Treg numbers among PBMCs from GVHD patients. Interestingly, the proportion of FOXP3+ cells among CD4+ cells more than doubled when GVHD cells were cocultured for 3 and 6 days with photodepletion-exposed versus untreated cells (Figure 4A), corresponding to a similar doubling of the absolute number of CD4+FOXP3+ cells (Figure 4B; P < .01). To investigate if this increase in the Treg population could be due to their proliferation, CD4+FOXP3+ cells were stained with Ki67. Interestingly, Tregs demonstrated the same level of Ki67 labeling whether they were cocultured with cells exposed or not to photodepletion (Figure 4C). EdU labeling confirmed that exposure to photodepletion cells did not promote DNA replication over that of untreated GVHD cells (Figure 4D). This lack of proliferative difference in the overwhelming majority of CD4+FOXP3+ Tregs strongly suggests that the postphotodepletion increase in the number of Tregs is not due to enhanced proliferation. To determine whether the phototreatment itself was responsible for conversion into a Treg phenotype, CD4+CD25− cells were isolated and treated with photodepletion. Photodepletion did not induce FOXP3 expression in CD4+CD25− cells (Figure 4E). However, the addition of photodepleted CD4+CD25+ cells to CD4+CD25− cells that had undergone staining with Mitotracker dye before the coculture led to an increased proportion of Mitotracker-positive cells expressing FOXP3. This was observed both in the presence of IL-2 alone or with additional allostimulation (P < .05; Figure 4F). Thus, these results demonstrate that the increase in Treg numbers reflects a conversion from CD4+FOXP3− into CD4+FOXP3+ cells, and that this conversion is induced by photodepleted CD4+CD25+ Tregs.24
Exposure of GVHD patient PBMCs to photodepletion cells increases Treg numbers. PBMCs from GVHD patients were cultured with untreated (untx) or photodepletion (PD)–exposed autologous PBMCs at a 1:1 effector:target ratio. At days 1, 3, and 6 of coculture, (A) percentages and (B) absolute numbers of FOXP3+ cells were determined by flow cytometry (mean ± SEM of 5 individual experiments). Black lines correspond to the addition of untreated PBMCs, and grey lines correspond to the addition of photodepletion-exposed PBMCs. (C) Treg proliferation was evaluated by measuring Ki-67 expression (mean ± SEM) and (D) EdU incorporation (representative example) in CD4+FOXP3+ cells after the coculture of GVHD patient cells with untreated (clear) or photodepletion-exposed (filled bars) GVHD PBMCs (3 independent experiments). (E) CD4+CD25− cells were isolated and exposed (or not) to photodepletion. Three days after photodepletion, surviving cells were characterized for the presence of FOXP3. (F) CD4+CD25− cells were isolated and stained with MitoTracker Deep Red. These cells were then cultured alone or with photodepleted CD4+CD25+ cells, in the presence of IL-2, or irradiated histoincompatible PBMCs and IL-2. (G) PBMCs from GVHD patients were untreated or exposed to photodepletion in the presence or absence of 50μM verapamil. Survival of CD4+ cells according to CD25 expression levels was assessed by 7-AAD staining. (H) GVHD PBMCs either untreated (clear), exposed to photodepletion alone (filled), or exposed to photodepletion in the presence of verapamil (striped bars) were then incubated with untreated autologous PBMCs, and proliferation was assessed by measuring 3H-thymidine incorporation. Bars represent mean ± SEM of 3 independent experiments. **P < .01 and ***P < .001 vs untreated targets alone (0:1) cells.
Exposure of GVHD patient PBMCs to photodepletion cells increases Treg numbers. PBMCs from GVHD patients were cultured with untreated (untx) or photodepletion (PD)–exposed autologous PBMCs at a 1:1 effector:target ratio. At days 1, 3, and 6 of coculture, (A) percentages and (B) absolute numbers of FOXP3+ cells were determined by flow cytometry (mean ± SEM of 5 individual experiments). Black lines correspond to the addition of untreated PBMCs, and grey lines correspond to the addition of photodepletion-exposed PBMCs. (C) Treg proliferation was evaluated by measuring Ki-67 expression (mean ± SEM) and (D) EdU incorporation (representative example) in CD4+FOXP3+ cells after the coculture of GVHD patient cells with untreated (clear) or photodepletion-exposed (filled bars) GVHD PBMCs (3 independent experiments). (E) CD4+CD25− cells were isolated and exposed (or not) to photodepletion. Three days after photodepletion, surviving cells were characterized for the presence of FOXP3. (F) CD4+CD25− cells were isolated and stained with MitoTracker Deep Red. These cells were then cultured alone or with photodepleted CD4+CD25+ cells, in the presence of IL-2, or irradiated histoincompatible PBMCs and IL-2. (G) PBMCs from GVHD patients were untreated or exposed to photodepletion in the presence or absence of 50μM verapamil. Survival of CD4+ cells according to CD25 expression levels was assessed by 7-AAD staining. (H) GVHD PBMCs either untreated (clear), exposed to photodepletion alone (filled), or exposed to photodepletion in the presence of verapamil (striped bars) were then incubated with untreated autologous PBMCs, and proliferation was assessed by measuring 3H-thymidine incorporation. Bars represent mean ± SEM of 3 independent experiments. **P < .01 and ***P < .001 vs untreated targets alone (0:1) cells.
Treg survival relies on a functional P-glycoprotein transporter
We have previously shown that P-glycoprotein is not only responsible for the cellular efflux of native rhodamine, but also of its TH9402 derivative.11 To determine whether Tregs use P-glyco-protein to escape photodynamic elimination, patient cells were exposed to TH9402 photodepletion in the presence of the potent P-glycoprotein inhibitor, verapamil. Upon verapamil exposure, CD4+CD25+ cells accumulated greater amounts of TH9402 than cells incubated with TH9402 but without verapamil, as measured by flow cytometry (data not shown). Moreover, the addition of verapamil in the context of photodepletion led to a dramatic impairment in T-cell viability in CD4 T cells expressing high, intermediate, or low levels of CD25 (Figure 4G; verapamil + photodepletion vs photodepletion only, n = 3, P < .001; compilation not shown). Most importantly, verapamil-induced TH9402 cell loading before illumination completely abrogated the ability of photodepletion cells to inhibit GVHD target cell proliferation (Figure 4H). Therefore, these results demonstrate that Tregs survived the photodepletion procedure because they harbor a functional P-glycoprotein transporter. In addition, the fact that verapamil caused massive death of photodepletion-treated cells, but did not impair target cell proliferation, implies that the photodepletion inhibitory effect is not caused by toxic mediators released from dying cells after photodepletion, but is rather actively mediated by cells surviving photodepletion.
Increased inhibitory cytokine secretion after exposure to photodepletion cells
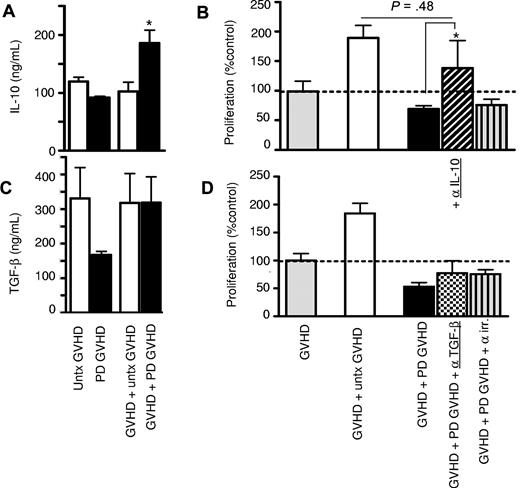
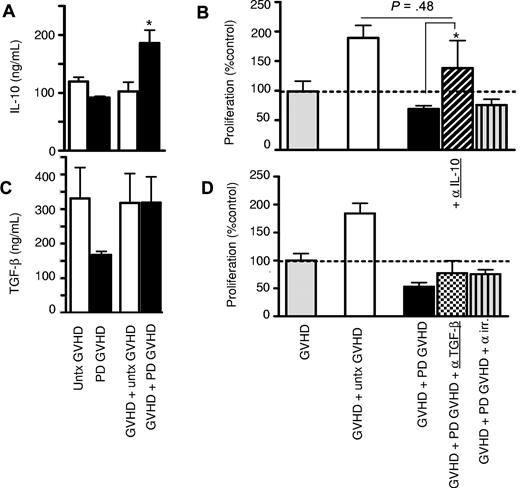
To determine which mechanism is utilized by Tregs from GVHD patients to exert their inhibitory activity, the involvement of the crucial Treg inhibitory cytokines, IL-10 and TGF-β, was assessed. IL-10 levels increased significantly when photodepletion cells were added to untreated GVHD cells (P < .05; Figure 5A). IL-10 involvement was confirmed in neutralization experiments where incorporation of anti–IL-10 mAbs resulted in a clear loss of the inhibitory effect (Figure 5B and supplemental Figure 2A). In contrast, soluble TGF-β levels failed to increase upon the addition of photodepletion cells (Figure 5C). In addition, mAb neutralization of TGF-β did not have any effect on proliferation inhibition (Figure 5D), confirming the lack of TGF-β involvement, whether in soluble or membrane form, in photodepletion-induced Treg activity. We also observed no changes in the levels of IFN-γ and TNF-α upon exposure to untreated or photodepletion cells (supplemental Figure 2B-C). We therefore conclude that photodepletion-preserved Tregs exert their inhibitory function through enhanced secretion of IL-10, but not TGF-β, IFN-γ, or TNF-α.
Increased IL-10 secretion after culture of photodepletion cells with untreated GVHD patient cells. GVHD patient PBMCs were cocultured with equal numbers of autologous untreated (clear) or photodepletion-exposed (filled bars) PBMCs. After 3 days of incubation, supernatants were collected, and (A) IL-10 and (C) soluble TGF-β levels were determined by ELISA. (B,D) GVHD patient PBMCs were cultured for 5 days with equal numbers of untreated (clear bars) or photodepletion-exposed PBMCs from the same patient in the absence (filled bars) or presence of neutralizing mAbs directed against IL-10 (diagonal), TGF-β (checked), or an irrelevant antigen (striped bars) of the same immunoglobulin G1 isotype, and cell proliferation was determined by measuring 3H-thymidine incorporation. Bars represent mean ± SEM of 3 independent experiments. *P < .05.
Increased IL-10 secretion after culture of photodepletion cells with untreated GVHD patient cells. GVHD patient PBMCs were cocultured with equal numbers of autologous untreated (clear) or photodepletion-exposed (filled bars) PBMCs. After 3 days of incubation, supernatants were collected, and (A) IL-10 and (C) soluble TGF-β levels were determined by ELISA. (B,D) GVHD patient PBMCs were cultured for 5 days with equal numbers of untreated (clear bars) or photodepletion-exposed PBMCs from the same patient in the absence (filled bars) or presence of neutralizing mAbs directed against IL-10 (diagonal), TGF-β (checked), or an irrelevant antigen (striped bars) of the same immunoglobulin G1 isotype, and cell proliferation was determined by measuring 3H-thymidine incorporation. Bars represent mean ± SEM of 3 independent experiments. *P < .05.
Crucial role of cell-cell contact and CTLA-4 in Treg-mediated inhibitory effect
Murine CD4+CD25+ Tregs require prior activation through their TCR to acquire full inhibitory potential, a step implying cell-cell contact and independent of cytokines.25 This raises questions about the initial steps leading to the activation and inhibitory activity of Tregs in patients with GVHD. To investigate the connection between IL-10 secretion and cell-cell contact events, effector cells were cultured while physically separated from target cells in Transwell plates. Preventing cell-cell contact between effector and target cells completely abrogated the inhibitory effect of photodepletion-treated cells and also abolished the previously observed increase in IL-10 secretion (Figure 6A-B). With CTLA-4 being the prime ligand on Tregs for members of the B7 costimulatory molecules and a crucial mediator of immune tolerance,26-28 its expression was measured on Tregs from patients with GVHD. We found that CTLA-4 was expressed on CD4+CD25+FOXP3+ cells after coculture with photodepletion cells (data not shown). In addition, Treg inhibition of proliferation was abrogated by anti–CTLA-4 antibodies (Figure 6C, P < .001, and supplemental Figure 2), but neither by anti-GITR nor by irrelevant isotype-matched antibodies. Interestingly, the addition of neutralizing antibodies directed against CTLA-4 completely abolished the 3-fold increase in the proportion (Figures 4A, 6D) and the more than doubling of the number (Figures 4B, 6E) of Tregs resulting from the addition of photodepletion cells to untreated GVHD cells (Figure 6D-E; P < .05). Thus, these results indicate that cell-cell contact is not only required for the inhibitory effect of photodepletion cells, but that it also represents an initiating event upstream of IL-10 secretion. In addition, these findings led to the identification of a crucial role for CTLA-4 in the inhibitory effect of photodepletion cells and in Treg production.
The inhibitory effect of photodepletion cells requires cell-cell contact and CTLA-4. (A) Photodepletion GVHD cells were physically separated from GVHD targets using Transwell plates to prevent cell-cell contact, but not cytokine interaction. After 5-day cultures, proliferation of target cells exposed to untreated (clear) versus Photodepletion-treated (filled bars) cells was measured in a 3H-thymidine incorporation assay, and (B) IL-10 levels were measured in the supernatants using ELISA. Bars represent mean ± SEM of 3 independent experiments. (C) PBMCs from GVHD patients were cultured with untreated (clear bars) or photodepletion-exposed PBMCs from the same patient for 5 days in the absence (filled bars) or presence of mAbs directed against CTLA-4, GITR, or an irrelevant antigen. Proliferation in each of the above conditions was determined by measuring 3H-thymidine incorporation (mean ± SEM). (D) FOXP3 expression in CD4+ cells was evaluated by flow cytometry after GVHD patient cells were cultured with autologous untreated cells, photodepletion-treated cells, or photodepletion-treated cells with anti–CTLA-4 mAbs. Results are expressed according to (D) the proportion of FOXP3+ cells within CD4+ cells and (E) the absolute number of CD4+FOXP3+ cells (results are shown as mean ± SD). *P < .05 and ***P < .001.
The inhibitory effect of photodepletion cells requires cell-cell contact and CTLA-4. (A) Photodepletion GVHD cells were physically separated from GVHD targets using Transwell plates to prevent cell-cell contact, but not cytokine interaction. After 5-day cultures, proliferation of target cells exposed to untreated (clear) versus Photodepletion-treated (filled bars) cells was measured in a 3H-thymidine incorporation assay, and (B) IL-10 levels were measured in the supernatants using ELISA. Bars represent mean ± SEM of 3 independent experiments. (C) PBMCs from GVHD patients were cultured with untreated (clear bars) or photodepletion-exposed PBMCs from the same patient for 5 days in the absence (filled bars) or presence of mAbs directed against CTLA-4, GITR, or an irrelevant antigen. Proliferation in each of the above conditions was determined by measuring 3H-thymidine incorporation (mean ± SEM). (D) FOXP3 expression in CD4+ cells was evaluated by flow cytometry after GVHD patient cells were cultured with autologous untreated cells, photodepletion-treated cells, or photodepletion-treated cells with anti–CTLA-4 mAbs. Results are expressed according to (D) the proportion of FOXP3+ cells within CD4+ cells and (E) the absolute number of CD4+FOXP3+ cells (results are shown as mean ± SD). *P < .05 and ***P < .001.
Injection of photodepletion-exposed PBMCs increases the proportion of Tregs in GVHD patients
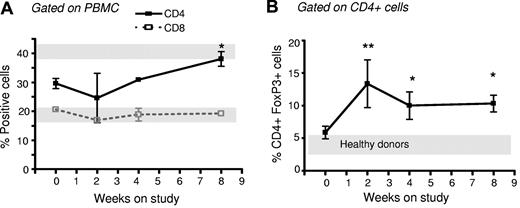
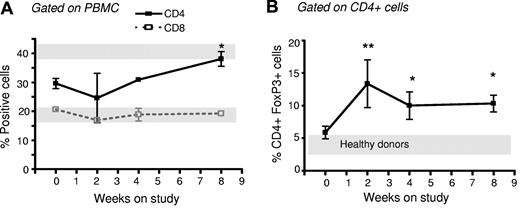
To determine whether the increased number of Tregs documented in our studies performed ex vivo would translate into an increase in Treg numbers in patients with chronic GVHD treated with photodepletion cells, we measured Treg numbers in 5 patients enrolled in a TH9402 photopheresis clinical trial. Patients were males, with a mean age of 47.8 years old (range, 39-58), and had undergone a myeloablative (busulfan and cyclophosphamide [n = 2] or total body irradiation with cyclophosphamide [n = 2] or melphalan and etoposide [n = 1]) allogeneic transplant with human leukocyte antigen–matched sibling donors. These patients had chronic GVHD refractory to at least 2 immune suppressors, including steroids, and underwent weekly leukapheresis and photodepletion using TH9402 under a clinical protocol (unpublished observation). PBMCs were analyzed at 0, 2, 4, and 8 weeks of treatment for their CD4+, CD8+, and CD4+FOXP3+ content. The proportion of CD4+ and CD8+ cells remained stable, except for a slight increase in CD4+ cells after 8 weeks of treatment (Figure 7A; P < .05). However, the number of Tregs in these patients' peripheral blood increased rapidly after 2 weeks and remained above pretreatment levels and also healthy donor levels until week 8 (P < .05; Figure 7B). Interestingly, these 5 patients also demonstrated some improvement in their GVHD. Indeed, complete responses were observed in the mouth, gastrointestinal tract, and skin, with 1 partial and 1 minimal response, the latter in lung GVHD. The increase in Tregs observed in vivo in GVHD patients suggests that reinfused Tregs can survive in vivo.
Increased proportion of CD4+FOXP3+ cells in GVHD patients receiving photodepletion cells. Five patients suffering from corticosteroid-refractory GVHD underwent weekly lymphophereses. Each cell collection was exposed to photodepletion and reinfused to the patient. Patient blood samples were obtained on weeks 0, 2, 4, and 8 for flow cytometric analysis of (A) CD4+ cells and CD8+ cells and (B) FOXP3+ cells within the CD4+ subpopulation. For comparison, gray bars indicate results from 5 healthy donors (mean ± SEM). Mean ± SEM of results from 5 patients; *P < .05 and **P < .01.
Increased proportion of CD4+FOXP3+ cells in GVHD patients receiving photodepletion cells. Five patients suffering from corticosteroid-refractory GVHD underwent weekly lymphophereses. Each cell collection was exposed to photodepletion and reinfused to the patient. Patient blood samples were obtained on weeks 0, 2, 4, and 8 for flow cytometric analysis of (A) CD4+ cells and CD8+ cells and (B) FOXP3+ cells within the CD4+ subpopulation. For comparison, gray bars indicate results from 5 healthy donors (mean ± SEM). Mean ± SEM of results from 5 patients; *P < .05 and **P < .01.
Discussion
In this study, we identified a novel photodepletion strategy with clinical potential for the treatment of patients with chronic GVHD. Indeed, we uncovered photodepletion conditions using TH9402, with the ability to specifically eliminate host-reactive T cells and preserve CD4+CD25+FOXP3+ T cells with Treg activity in PBMCs from patients with chronic GVHD. Such Tregs present in photodepletion-treated PBMCs at the time of reinfusion have the potential to suppress the pro-GVHD activity of patient T cells that have not been exposed to photodepletion. Tregs from patients or healthy individuals escape photodepletion-induced elimination via P-glycoprotein–mediated dye efflux. Notably, Treg generation and activity required CTLA-4 binding and was completely abrogated by the prevention of cell-cell contact between photodepletion cells and untreated patient cells. We also showed that IL-10 secretion is a prime mechanism responsible for the inhibition of GVHD patient cell proliferation, while other mediators, such as TGF-β and GITR, were not involved. Finally, we provide data supporting the rapid expansion of Tregs in patients reinfused with autologous photodepletion-treated PBMCs.
The photodepletion inhibitory effect on untreated cell proliferation cannot be ascribed solely to the elimination of host-reactive T cells. The fact that TH9402 photodepletion preserved functional CD4+CD25+FOXP3+ cells clearly positioned Tregs as likely effectors of this inhibitory effect. In addition, depletion of CD4+CD25+ cells resulted in complete loss of the inhibitory effect of photodepletion-exposed cells on both MLR-activated and GVHD patient cells, a finding providing further support to the implication of Tregs. Moreover, the fact that the addition of cells killed by photodepletion did not impair the capacity of GVHD cells to proliferate indicated that the decreased proliferation was not attributable to a bystander effect resulting from soluble mediators or death signals from dying cells. Finally, adding back CD4+CD25+ cells completely restored the inhibition of proliferation lost upon the removal of Tregs. We thus conclude that the ability of photodepletion-treated PBMCs from GVHD patients to inhibit the proliferation of untreated CD4 T cells is due to Treg enrichment.
Although a few human29-31 and murine9,32,33 studies have provided evidence for the implication of Tregs in the inhibition of alloreactivity in ECP using the photosensitizer 8-methoxypsoralen (8-MOP), this is the first study to look closely at the cellular and molecular mechanism by which Tregs surviving photodepletion can exert such cellular inhibition in the context of human chronic GVHD. The 2 most common hypotheses to explain Treg modulation of immune responses imply either secretion of the anti-inflammatory cytokines, IL-1034 and TGF-β,35 or cell-cell contact mediated by CTLA-4 or membrane-bound TGF-β.36-38 In the present study, we show that the addition of TH9402 photodepletion cells to untreated GVHD cells leads to increased IL-10 secretion, which causes their inhibitory effect, as confirmed by IL-10 blocking experiments. As opposed to several studies where TGF-β was shown to play a pivotal role in the inhibitory effect of Tregs,38 in the present study, neutralization of TGF-β did not alter photodepletion-induced inhibition. However, our results are consistent the observation by Kullberg et al that CD4+ Tregs from SMAD3−/− animals, whose TGF-β signaling pathway is impaired, are able to generate an immunosuppressive effect.39 Our study also demonstrates that IL-10 secretion is cell-cell–contact dependent, indicating that IL-10 secretion does not result from a direct effect of photodepletion on PBMCs. With CTLA-4 being a receptor of major importance in Treg cell-cell contact and in cytokine-dependent inhibition,37,40 we investigated its role in TH9402 photodepletion–induced immune suppression. We found that CTLA-4 neutralization abrogated the inhibitory effect of photodepletion cells on the proliferation of host-reactive T cells. This finding is in agreement with the observation of decreased suppressive capacity in CTLA-4–deficient murine Tregs.41 In addition, Bodor et al have shown in a murine model that CTLA-4 could mediate its inhibitory effect through induced cAMP early responder, a mechanism involving T-T cell interactions.42 Our results also associated CTLA-4 inhibition with a reduced frequency of Tregs. It will therefore be interesting to evaluate whether strategies to promote CTLA-4 signaling on Tregs will lead to the expansion of this cell population.
There are 2 main types of CD4 Tregs, FOXP3+ Tregs, and IL-10–producing type 1 Tregs (Tr1 cells).24 Our work clearly shows that the photodepletion of PBMCs leads to an enrichment of FOXP3+ Treg cells. However, the fact that the Treg activity of photodepletion-treated cells is IL-10 dependent suggests that Tr1 may contribute to this Treg activity. In line with this idea, evidence suggests that human FOXP3+ Tregs induce the development of Tr1 cells in vitro.43 We could not directly address this question herein because there are no known specific cell-surface markers of Tr1 cells.24 We speculate that Treg cells found in GVHD patients are of extrathymic origin. Tr1 cells always arise in the periphery when naïve CD4+ T cells are activated by tolerogenic antigen-presenting cells.44 Although FOXP3+ Treg cells can be generated in the thymus and the periphery, several points lead us to speculate that those found in PBMCs from GVHD patients are mostly, if not exclusively, of extrathymic origin. First, patients with active chronic GVHD present important thymic atrophy, decreased thymic output, and peripheral lymphopenia.45,46 Second, the factors responsible for the generation of FOXP3+ Tregs in the periphery coalesce in GVHD patients: Lymphopenia-induced homeostatic T-cell proliferation and production of TH1 cytokines enhances the generation of FOXP3+ T cells in the periphery.47-49 Generation of Tregs in the periphery is limited because CD4 Tregs are dependent on IL-2 produced by other CD4+ T cells.50 Thus, the CD4+ Treg population and the non-naïve IL-2–producing CD4 T-cell population control each other. We therefore propose that supplementation with IL-2 after photodepletion could lead to a further expansion of Tregs without the accumulation of CD4 effector T cells.
Based on the results of the present study, a multicenter clinical trial of ECP using TH9402 for the treatment of patients with steroid-refractory chronic GVHD has been initiated. In this study, circulating lymphocytes were collected by leukapheresis, exposed ex vivo to photodepletion, and reinfused into the patient. We surmised that the increase in Treg numbers observed in vitro would translate into the generation of Tregs in vivo. To test this hypothesis, we evaluated Treg populations in these patients and found that it increased at all 3 time points evaluated after treatment initiation, suggesting that results from our studies performed ex vivo are relevant to the biology of Tregs in GVHD patients and translate into population changes in vivo. Treg increases were also observed after ECP using 8-MOP as a photosensitizer. However, in these studies, the increase in Tregs was observed after approximately 9 weeks (6 treatment cycles), while TH9402 treatment led to an increase in Tregs as early as 2 weeks after treatment initiation.30 Whether this rapid rise in Tregs could be explained by the infusion of Tregs preserved after TH9402 photodepletion, or by the generation of new Tregs, remains to be determined. This result is in line with the ability of ECP to reverse experimental GVHD through a Treg-dependent mechanism in an animal model of acute GVHD,9 and is most encouraging for the implementation of a TH9402-based ECP process that would rely both on the elimination of immunoreactive cells and Treg generation. The latter may have particularly important clinical implications, because the proportion of Tregs in peripheral blood was found to correlate with the incidence of GVHD.17 Moreover, the demonstration, in the present study, that photodepletion has an impact on both endogenous host-reactive T cells and Tregs could have important biologic implications. Indeed, immunoreactivity from noncirculating lymphocytes that inevitably escape exposure to photodepletion could be downmodulated immediately by Tregs surviving the photodepletion process and reinfused into the patient and later by Tregs generated in vivo after cell–cell contact with photodepleted cells. This could explain why ECP has beneficial effects, although it targets only 15% or less of total body lymphocytes. Such a strategy may represent a significant advance for the treatment of patients with chronic GVHD and other immunologic disorders.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Francine Foss, Ronan Foley, Irwin Walker, Harry Atkins, Jean Roy, Silvy Lachance, Thomas Kiss, Lambert Busque, Guy Sauvageau, and Marie Detrait for their help in the conception and implementation of the Phase I/II clinical trial of TH9402 ECP for the treatment of steroid-refractory chronic GVHD; Drs Martin Guimond and José Cohen for their insightful comments on the manuscript; and Jean Morin and the members of the Cellular Therapy Laboratory, Apheresis and Stem Cell Transplantation Units in Montreal, Ottawa, and Vancouver for their help with the procurement and preparation of human samples, as well as treatment of patients.
This study was supported by Kiadis Pharma and the Fonds de la recherche en sante du Quebec.
Authorship
Contribution: J.-P.B., G.K., and D.C.R. designed the research; J.P.B. and M.R. performed the research; C.T. and M.R. contributed data; J.P.B. and G.K. analyzed data; C.S., S.C., D.S.A., D.H., R.M.E., and D.C.R. provided patient cells and reagents; J.P.B., G.K., and D.C.R. wrote the manuscript; and D.C.R., C.S., S.C., R.M.E., and C.P. edited the manuscript.
Conflict-of-interest disclosure: D.C.R. has received research grant support from Kiadis Pharma. R.M.E. is an employee of Kiadis Pharma. The remaining authors declare no competing financial interests.
Correspondence: Denis Claude Roy, Division of Hematology-Oncology, Hopital Maisonneuve-Rosemont Research Center, 5415 l'Assomption Blvd, Montreal, Quebec H1T 2M4, Canada; e-mail: denis-claude.roy@umontreal.ca.