Abstract
The survival of diffuse large B-cell lymphoma patients varies considerably, reflecting the molecular diversity of tumors. In view of the controversy whether cytologic features, immunohistochemical markers or gene expression signatures may capture this molecular diversity, we investigated which features provide prognostic information in a prospective trial in the R-CHOP treatment era. Within the cohort of DLBCLs patients treated in the RICOVER-60 trial of the German High-Grade Lymphoma Study Group (DSHNHL), we tested the prognostic impact of IB morphology in 949 patients. The expression of immunohistochemical markers CD5, CD10, BCL2, BCL6, human leukocyte antigen (HLA)–DR, interferon regulatory factor-4/multiple myeloma-1 (IRF4/MUM1), and Ki-67 was assessed in 506 patients. Expression of the immunohistochemical markers tested was of modest, if any, prognostic relevance. Moreover, the Hans algorithm using the expression patterns of CD10, BCL6, and interferon regulatory factor-4/multiple myeloma-1 failed to show prognostic significance in the entire cohort as well as in patient subgroups. IB morphology, however, emerged as a robust, significantly adverse prognostic factor in multivariate analysis, and its diagnosis showed a good reproducibility among expert hematopathologists. We conclude, therefore, that IB morphology in DLBCL is likely to capture some of the adverse molecular alterations that are currently not detectable in a routine diagnostic setting, and that its recognition has significant prognostic power.
Introduction
Diffuse large B-cell lymphomas (DLBCLs) can be subdivided based on cytolologic appearance (eg, centroblastic [CB] vs immunoblastic [IB] morphology), their site of origin (nodal vs extranodal), or in relation to the specific clinical background in which they arise (eg, state of immunodeficiency).1 As a consequence of this heterogeneity, there is marked variation in the response of DLBCL patients to current treatment approaches that is reflecting the considerable molecular diversity of these tumors. In recent years, gene expression profiling studies provided a host of information about the underlying molecular mechanisms of DLBCL and defined gene expression signatures that are related to the cell of origin of the tumors or to important features of the microenvironment.2-5 Specifically, the distinction between the germinal center B-like DLBCL subtype (GCB DLBCL) and activated B-like DLBCL subtype (ABC DLBCL) had important prognostic implications in the cyclophosphamide, vincristine, doxorubicin, and prednisolone (CHOP) treatment era with better outcome for GCB DLBCL patients.4 In an attempt to translate the multigene classifiers for the GCB/ABC DLBCL distinction into more practicable and clinically feasible diagnostic tests, immunohistochemical (IHC) signatures were developed to capture the GCB/ABC DLBCL or GCB/nonGCB DLBCL distinction. Of these, the classifier developed by Hans and colleagues6 using the markers cluster of differentiation (CD)10, B-cell lymphoma (BCL)–6, and interferon regulatory factor-4/multiple myeloma-1 (IRF4/MUM1) gained broad consideration and was widely applied to large cohorts of DLBCL tumors. Subsequent studies, however, yielded conflicting results regarding its prognostic power. These were attributed to varying selection criteria for the DLBCL cohorts investigated and to significant interlaboratory variation of technical aspects as well as interobserver variation in the interpretation of IHC results.7,8 These controversies extended into the rituximab (R)–CHOP treatment era raising the question whether the GCB/nonGCB distinction in DLBCL is still relevant when modern treatment modalities using combined immunochemotherapy are applied. However, a recent gene expression profiling study3 of R-CHOP treated DLBCL patients provided evidence that under rituximab patients with GCB DLBCL tumors have better survival in comparison to patients with ABC DLBCL tumors. It remains an important goal, therefore, to develop a robust diagnostic test for the GCB/nonGCB DLBCL subtype distinction, and outcome prediction in general; that is, applicable in clinical routine and reproducible in terms of interobserver agreement.
Because most of the previously published studies were performed in DLBCL cohorts outside of clinical trials, the present study sought to clarify the relevance of morphologic and IHC biomarkers in R-CHOP treated patients. This was performed on the basis of the rituximab with CHOP over age 60 years (RICOVER-60) trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) that included 1222 rituximab-treated as well as nonrituximab-treated patients over 60 years of age.9 For a complete list of DSHNHL participants, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Methods
Patients and samples
All cases analyzed in this study were enrolled in the prospective randomized multicenter clinical trial RICOVER-60 of the DSHNHL,9 in which 1222 patients over 60 years of age with CD20-positive aggressive BCLs were randomly assigned to 6 or 8 cycles of CHOP-14 with or without addition of 8 cycles rituximab. This clinical trial was conducted in accordance with the Helsinki declaration and the protocol had been approved by the ethics review committee of each participating center.
Diagnostic samples from the study patients were reviewed by expert hematopathologists in the 6 German reference centers for lymph node pathology. Morphologic analysis was carried out on tissue sections stained with hematoxylin and eosin (HE) and Giemsa. Subtyping followed the definitions of the 2008 WHO classification.1 Inclusion criteria for the present study were a reference diagnosis of DLBCL (949 patients), and the availability of formalin-fixed and paraffin-embedded material for tissue microarray construction (506 cases).
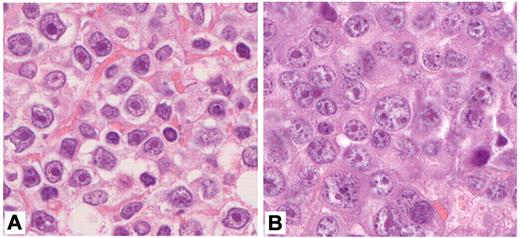
The exact criteria for the diagnosis of IB lymphomas were established during panel meetings of the entire pathology group carried out regularly 3 times a year and had a high consistency. In general, we followed the classical definition given by Lennert.10,11 Specifically, this definition requires that IB lymphomas be mainly composed of immunoblasts; that is, cells with blastic appearance demonstrating a small- to medium-sized rim of basophilic cytoplasm and large nuclei with light chromatin and a prominent singular nucleus (Figure 1A), and that cells typical of the germinal center (ie, centroblasts and variants of centrocytes) should comprise less than 10% of the entire cell population. In practice, however, the number of classical immunoblasts in these lymphomas turned out to be smaller than 30% in some cases, which were nevertheless classified as IB by the panel. In those cases, we observed a sometimes substantial population of cells with plasmablastic or plasmacytoid features as exemplified in Figure 1B.10,11 Although these cells with plasmablastic or plasmacytoid features had been included in the classical description by Lennert as an integral part of IB lymphomas, the panel deliberately broadened the definition of IB so that these lymphomas should consist (in the majority of cells, that is > 90%) of typical IB and/or plasmablastic/plasmacytoid cells.
Cytomorphology of immunoblastic lymphoma. (A) IB lymphoma predominantly harboring large cells with abundant, deeply basophilic cytoplasm, large vesicular nuclei and a large central solitary nucleolus (100× objective, total magnification ×1000). (B) IB lymphoma with plasmablastic features. Note that the predominant cell is large, with abundant, basophilic cytoplasm and eccentric, round to oval nuclei differing in size and a solitary or several paracentric nucleoli (100× objective, total magnification ×1000). Images have been performed with a Zeiss Axiophot Microscope, a Jenoptik ProgRes CF camera, and the ProgRes CapturePro 2.6 software package for image processing (Jenoptik).
Cytomorphology of immunoblastic lymphoma. (A) IB lymphoma predominantly harboring large cells with abundant, deeply basophilic cytoplasm, large vesicular nuclei and a large central solitary nucleolus (100× objective, total magnification ×1000). (B) IB lymphoma with plasmablastic features. Note that the predominant cell is large, with abundant, basophilic cytoplasm and eccentric, round to oval nuclei differing in size and a solitary or several paracentric nucleoli (100× objective, total magnification ×1000). Images have been performed with a Zeiss Axiophot Microscope, a Jenoptik ProgRes CF camera, and the ProgRes CapturePro 2.6 software package for image processing (Jenoptik).
Reproducibility study
To establish the grade of robustness of the classification of a DLBCL as IB lymphoma, we sought to clarify the degree of reproducibility of this diagnosis among hematopathologists (interobserver variability). To this end, 80 cases were selected from the cohort of the German Molecular Mechanisms in Malignant Lymphomas (MMML) studies preclassified by the German lymphoma reference panel, of which 40 had been classified as IB lymphomas and 40 as nonIB lymphomas. Three pathologists blinded to the panel's review diagnosis independently classified all cases as IB or nonIB cases, and the interobserver agreement was assessed.
TMA construction
IHC profiling was performed on tissue microarrays (TMAs) constructed at each reference center introducing 1 to 5 0.6-mm cores per case in a total of 25 recipient blocks containing 25 to 60 cases per TMA block. Altogether, 506 cases were represented on the TMAs. From these blocks, 3-μm sections were cut and sent to 1 defined staining center for each antibody.
IHC staining and scoring
The IHC panel consisted of antibodies against CD20, CD10, CD3, CD5, BCL2, BCL6, IRF4/MUM1, human leukocyte antigen (HLA)–DR, and Ki-67. The primary antibodies, suppliers, and staining conditions are listed in Table 1.
All immunostainings were independently evaluated by 3 (of 6) experienced hematopathologists from the contributing reference centers in Würzburg, Kiel, Berlin, Frankfurt, Lübeck, and Ulm who were blinded for clinical details. Stainings for CD20, CD3, CD10, and HLA-DR were scored as positive or negative, and stainings for CD5, BCL2, IRF4/MUM1, and BCL6 were scored in a semiquantitative manner indicating the percentage of positive tumor cells: No staining (score 1), 5%-25% (score 2); 26%-50% (score 3); 51%-75% (score 4); > 75% (score 5). For Ki-67 (MIB1), score 5 was defined at 76%-90%, and an additional score 6 was introduced (> 90%). Whenever individual cores of a given case showed non-concordant results, the core with the highest number of positive cells was scored. For each individual case, an identical scoring regarding the groups (negative, positive) by the 3 independent investigators or, in case of no unanimous agreement, the identical votes of 2 observers were recorded as the result. Otherwise, a case was recorded as “not evaluable” for a given antigen.
Statistical analysis
Event-free survival (EFS) was defined as the time from randomization to disease progression, start of salvage treatment, additional (unplanned) treatments, relapse, or death from any cause. Overall survival (OS) was defined as the time from randomization to death of any cause. EFS and OS were estimated according to Kaplan-Meier.12
To determine the cutoff points for statistical analysis discriminating positive and negative cases, the sample size within score groups, the results of log rank tests for different scenarios with different definitions of positivity and results from single observers were taken into account to decide which cutoff points were most suitable.
In the univariate analyses, log rank tests were performed. The International Prognostic Index (IPI; age older than 60 years, lactate dehydrogenase [LDH] > normal, Easter Cooperative Oncology Group [ECOG] > 1, stage III/IV, and extranodal involvement > 1) is the current gold standard for prognostic stratification in DLBCL, and therefore proportional hazard models for each of the selected parameters were separately adjusted for the factors of the IPI. Before modeling, we excluded relevant correlations of the respective parameters with IPI factors (ie, Pearson correlation coefficient > 0.7). Relative risks with 95% confidence intervals (CI) and P values are presented. The results for the immunohistochemical markers are shown in a forest plot. For the correlation of marker expression with qualitative data (IPI score groups and morphologic subtypes) and to check whether there were differences regarding patients characteristics, we used the χ2 test and, if required, Fisher exact test. Because of the descriptive nature of these comparisons, the P values were not adjusted for multiple comparisons and the significance level was P = .050. For many of the IHC markers we observed a variable number of cases failing to yield reliable staining results and, accordingly, the latter were excluded from analysis. Thus, the number of evaluable cases varied from one marker to another. This also decreased the number of cases that were available for the GCB/nonGCB subgrouping (n = 352) and for the Cox model that included BCL2 and BCL6 (n = 348). To assess the concordance between different observers regarding IHC and morphology (IB vs nonIB), a generalized statistic kappa was used.13 Statistical analyses were done with SPSS 15.0 and R-2.7.1 (package ‘irr’ Version 0.70).
Results
Patient characteristics
Table 2 provides an overview regarding the clinical parameters of all 949 DLBCL patients enrolled in the RICOVER-60 trial. Table 2 also details clinical data of patients according to the morphologic subgroups of their tumors, and of those 506 patients, in whom immunohistochemistry could be performed on TMAs, as well as data on 166 patients with tumors classified as GCB DLBCL and 186 patients with tumors classified as nonGCB DLBCL. With regard to clinical parameters, the DLBCL cohort that was amenable to IHC analyses (n = 506) closely matched the entire study population of the RICOVER-60 trial. There were no significant differences between CB and IB patients with respect to the IPI factors and to the IPI score in general. As can be seen from the table, nonGCB patients differed from GCB patients in some items of clinical presentation, most notably for the IPI factors stage III/IV (P = .016), more than 1 extranodal involvement (P = .032) and the IPI score (P = .013).
Morphologic DLBCL subtypes
Upon morphologic analysis, 516 cases (54.4%) were classified as the CB variant, and 70 cases (7.4%) as the IB variant.10,11 The anaplastic variant was diagnosed in 19 (2.0%) cases, primary mediastinal B-cell lymphoma (PMBL) in 13 (1.4%) DLBCL, and 18 (1.9%) DLBCL were classified as T-cell/histiocyte rich large B-cell lymphoma (THRLBCL). A diagnosis of DLBCL without further specification was given in 313 (33.0%) cases.
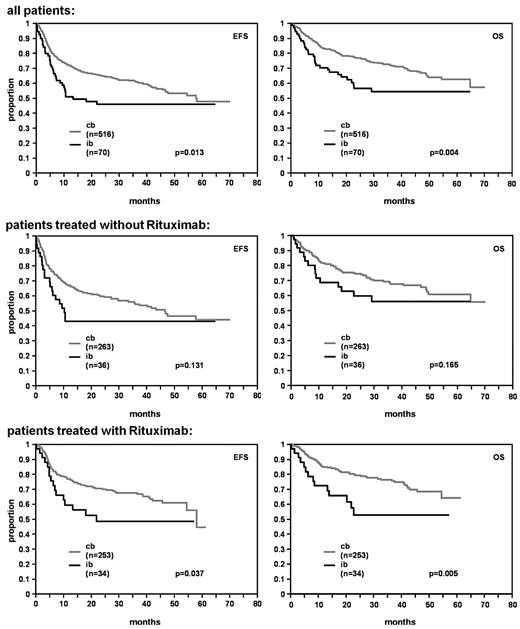
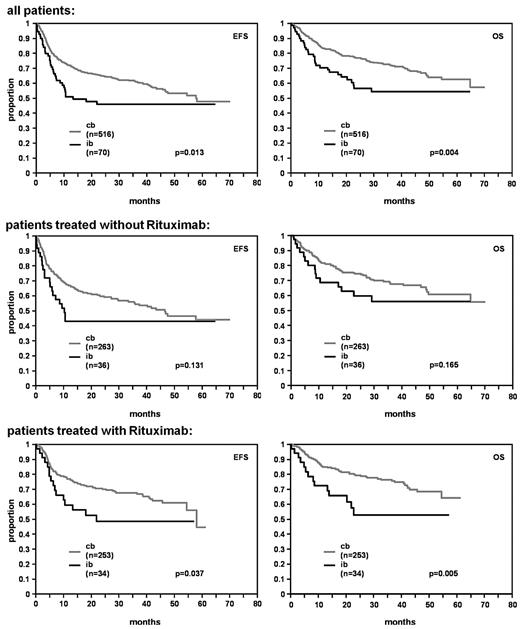
In univariate analysis, patients with the IB subtype had a significantly lower CR/CRu rate in comparison to patients with CB subtype (62.9%; 95% confidence interval [CI]: 50.5-74.1 vs 76.7%; 95% CI: 72.9-80.3; P = .012). IB morphology predicted for an inferior prognosis with respect to both EFS and OS in the whole study population (P = .013 and P = .004, respectively; Figure 2).
EFS and OS for DLBCL patients with centroblastic (cb) and immunoblastic (ib) morphology.
EFS and OS for DLBCL patients with centroblastic (cb) and immunoblastic (ib) morphology.
The survival curves for all other subtypes of DLBCL closely matched the curve of CB lymphomas (data not shown).
In multivariate analysis adjusted for the factors of the IPI the IB subtype was an independent predictor for EFS (relative risk [RR] = 1.5, 95% CI: 1.0-2.1), P = .034) and OS (RR = 1.7, 95% CI: 1.2-2.6, P = .007) with relative risks in the order of the IPI factors (Table 3).
In multivariate analysis adjusted for the IPI factors including the factors IB versus CB, with versus without rituximab, and the interaction term of both there was no relevant interaction between morphologic subtype and rituximab for EFS and OS (EFS: RR = 1.1, 95% CI: 0.5-2.3, P = .753; OS: RR = 1.2, 95% CI: 0.5-2.8, P = .605).
Statistical analysis of the interobserver reliability study concerning the diagnosis of IB lymphoma revealed a reliable degree of robustness for the diagnosis of IB among experienced hematopathologists with a kappa coefficient of 0.64.
IHC analysis
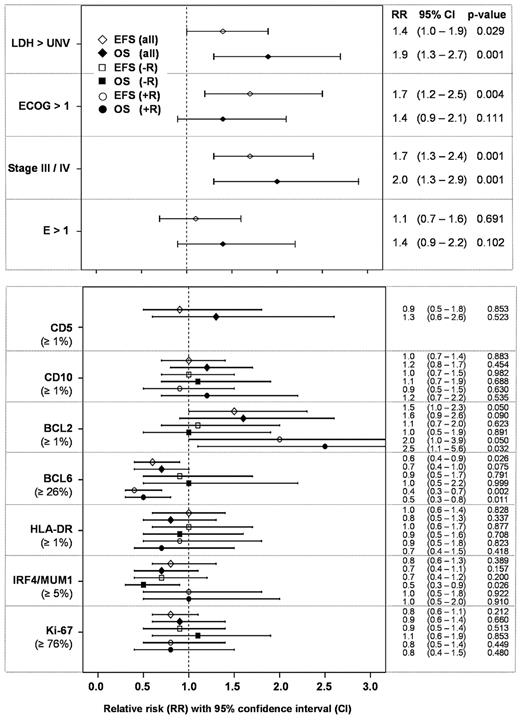
Table 4 summarizes the results of the IHC analysis. For comparison, the Cox model of the prognostic factors of the IPI is presented for EFS and OS in the upper panel of Figure 3. The IPI factors LDH, performance status ECOG, and stage were the strongest prognostic factors with relative risks ranging from 1.4 to 2.0 (for LDH > normal, ECOG > 1, and stage III/IV), and 0.7 to 0.5 (for LDH ≤ normal, ECOG ≤ 1, and stage < III), respectively.
EFS and OS for the IPI factors (n = 506) and for single IHC parameters adjusted for IPI factors within Cox models.
EFS and OS for the IPI factors (n = 506) and for single IHC parameters adjusted for IPI factors within Cox models.
The stainings for all IHC parameters were scored independently by 3 of 6 observers from the contributing reference centers. Regarding the assignment to the categories “not scored,” “negative,” and “positive” according to the respective cutpoints for the number of positive tumor cells, the kappa-values were: 0.64 (CD5), 0.80 (CD10), 0.77 (BCL2), 0.74 (BCL6), 0.78 (HLA-DR), 0.71 (IRF4/MUM1), and 0.61 (Ki-67). Thus, a satisfactory interobserver reliability was achieved.
The lower panel of Figure 3 displays the correlation between the expression levels of individual IHC markers with EFS and OS. The relative risk estimates with 95% confidence interval obtained from Cox models for single markers adjusted for the factors of the IPI are presented in a forest plot. The expression of BCL2 and BCL6 were the only markers to predict EFS in the entire study population. Their contribution to the model, however, was rather weak in comparison to the relative risks of the IPI factors. Of interest, expression of BCL6 predicted both superior EFS and OS in the patient cohort treated with rituximab. Similarly, BCL2 expression predicted shorter EFS and OS in R-CHOP, but not in CHOP-only treated patients. However, a formal test on interaction between rituximab treatment and BCL2 or BCL6 expression did not show a significant result for EFS (P = .252 and P = .068) and OS (P = .147 and P = .127). Including BCL2 and BCL6 in a multivariate Cox model adjusted for the IPI factors both yield a similar independent contribution regarding the relative risk estimates for EFS (RR(BCL2) = 1.5, 95% CI 0.9-2.4, P = .105; RR(BCL6) = 0.7, 95% CI 0.4-1.0, P = .042) and OS (RR(BCL2) = 1.5, 95% CI 0.9-2.7, P = .141; RR(BCL6) = 0.7, 95% CI 0.4-1.1, P = .131).
All other markers (CD5, CD10, HLA-DR, IRF4/MUM1, and Ki-67 proliferation index) failed to yield consistent prognostic information both with respect to EFS and OS, as well as in DLBCL cohorts treated with and without rituximab.
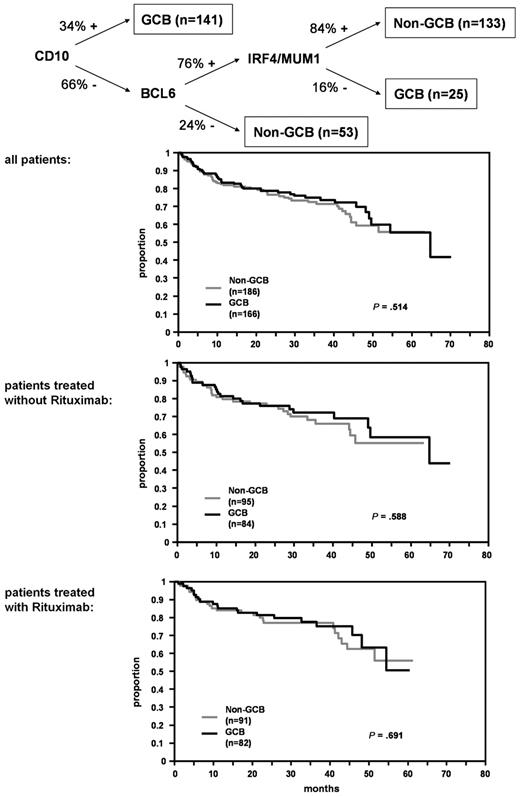
Analyses of GCB-type versus nonGCB-type DLBCL
To assign DLBCL cases to the GCB or nonGCB subgroups, we applied the classifier proposed by Hans et al6 that integrates the expression patterns of CD10, BCL6, and IRF4/MUM1 (Figure 4). In close approximation to the suggested cutpoints in the original publication, BCL6 and IRF4/MUM1 were regarded as positive, when at least 25% of tumor cells were stained. However, this classifier failed to discriminate prognostically relevant DLBCL subgroups within the RICOVER-60 trial for OS (Figure 4) and EFS (data not shown). Moreover, no differences were observed when DLBCL patients with and without rituximab treatment were analyzed separately. Multivariate Cox models adjusted for IPI factors showed no significant differences between NonGCB and GCB groups for EFS (relative risk [RR][all] = 1.2, 95% CI 0.8-1.7, P = .331; RR[without rituximab] = 1.1, 95% CI 0.7-1.7, P = .710; RR[with rituximab] = 1.3, 95% CI 0.8-2.2, P = .299) and OS (RR[all] = 0.9, 95% CI 0.6-1.4, P = .761; RR[without rituximab] = 0.9, 95% CI 0.5-1.6, P = .733; RR[with rituximab] = 1.0, 95% CI 0.5-1.8, P = .901). Finally, no prognostic subgroups (nonGCB: n = 101, GCB: n = 120) were discerned when the analysis was restricted to DLBCL with CB morphology only (data not shown). There was a significant association between the cytomorphologic subclassification of the tumors (CB/IB) and their grouping according to the Hans classificator, with almost all IB tumors falling into the nonGCB group (20/23 or 87.0%, P < .001). The IB effect is independent of the classification according to GCB and nonGCB.
Correlation of immunohistochemistry with the IPI score and morphology
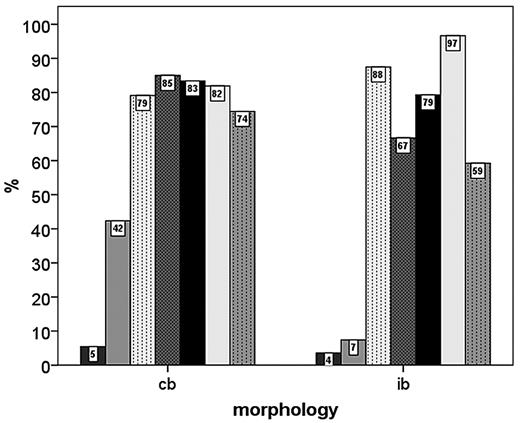
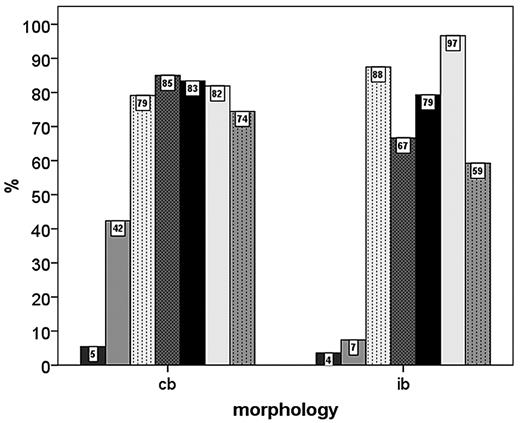
No significant correlations of IPI score groups (1-2 versus 3-5) with IHC features of the tumor cells were seen. On the IHC level, IB morphology was significantly associated with negativity for CD10 and BCL6 expression and positivity for IRF4/MUM1 expression. Interestingly, there was a trend toward higher Ki-67 scores in the CB subgroup compared with IB DLBCL (Figure 5).
Percentage of DLBCL patients with positivity for various IHC parameters in the centroblastic (cb) and immunoblastic (ib) subgroups.
Percentage of DLBCL patients with positivity for various IHC parameters in the centroblastic (cb) and immunoblastic (ib) subgroups.
Discussion
In the RICOVER-60 trial of the DSHNHL 949 DLBCL patients over 60 years were treated with 6 or 8 cycles of CHOP-14 therapy with or without 8 applications of rituximab.9 Within these patients, IB morphology of the tumor cells emerged as a significant adverse prognostic factor that retained its independent prognostic relevance in multivariate analysis even when the clinical IPI factors were included. The prognostic impact of IB morphology was prominent within the patient cohort treated with R-CHOP. In the subgroup of 506 patients in which immunohistochemistry was performed, the expression levels of markers including CD5, CD10, BCL2, BCL6, HLA-DR, IRF4/MUM1, and the Ki-67 proliferation index were of modest prognostic significance or not significant at all. Moreover, the widely used Hans classifier that, based on the expression of CD10, BCL6, and IRF4/MUM1, assigns DLBCL into the GCB and nonGCB DLBCL categories failed to show any prognostic significance in the entire cohort or in the patient subgroups treated with or without rituximab.
The gene expression-based distinction between GCB DLBCL and ABC DLBCL carried a prognostic impact in the CHOP-treatment era2,5 and appears to retain its prognostic value when DLBCL patients are treated with R-CHOP.3 However, when IHC algorithms were developed to translate the complex and multigene expression signatures into more practically feasible standard assays applicable to routine formalin-fixed, paraffin-embedded tumor tissues, results from retrospective analyses of various DLBCL cohorts were highly contradictory. In the CHOP-treatment era, the IHC classifier developed by Hans and colleagues,6 or slightly modified versions of it, predicted survival of DLBCL patients in some studies,6,14-19 but not in others.20-25 Likewise, results were contradictory in DLBCL cohorts treated with R-CHOP.17,26-28
Several reasons may account for these discrepancies. First, retrospective analyses of relatively small series of DLBCL patients with heterogeneous treatment modalities may have confounded the results. Moreover, patient series from single institutions may have been prone to selection biases. Second, technical shortcomings and interobserver variations in the interpretations of the staining results may also account for divergent results.7,8 Both shortcomings were conceptually overcome in the present study, in which DLBCL tumors from a large, prospective phase 3 trial were analyzed using a highly standardized staining and scoring procedure. In line with our previous report on a negative prognostic impact of IB morphology in a large prospective DLBCL trial from the CHOP treatment era (non-Hodgkin lymphoma-[NHL]-B1 and -B2 by the DSHNHL29 ), we now provide evidence that this morphologic parameter, which can be easily determined using standard hematoxylin and eosin staining, carries important prognostic information also in the R-CHOP treatment era. The prognostic impact of IB morphology in DLBCL has been debated since the publication of Engelhard and colleagues,30 and several authors raised doubt as to its utility as a prognostic marker, also questioning the reproducibility of the diagnosis. In other series, however, IB morphology turned out to represent a negative prognostic indicator.22,31 However, we have now, recapitulating the results of cytomorphologic evaluation in the German NHL-B1/B2 trial by the DSHNHL,29 provided evidence that IB morphology is an independent risk factor in DLBCL and continues to be in the Rituximab era. Furthermore, we have shown that the diagnosis of IB is reproducible among hematopathologists and altogether, therefore, can be viewed as a robust clinicopathologic marker in DLBCL.
In contrast, the most widely evaluated antigens in DLBCL are of minor value when it comes to risk assessment of DLBCL patients treated with R-CHOP. Although the expression levels of BCL2 and BCL6, in line with previous studies,32,33 showed modest associations with inferior (BCL2) or improved (BCL6) outcome in our study, their impact was modest in multivariate analysis when the classical IPI factors were included. In addition, HLA-DR positivity was associated with a better outcome within IPI-score groups 1 and 2, but not within IPI-score groups 3 to 5. However, these results are based on a very small sample size. Nevertheless, HLA-DR was the most prominent prognostic factor within the NHL-B1/B2 trial, including young low risk patients (NHL-B1) and elderly patients from all risk groups (NHL-B2).29 Importantly, the IHC classifier developed by Hans6 failed to be of prognostic impact in the CHOP arm, in the R-CHOP arm, and in the entire study population. We thus conclude that the IHC assignment of a DLBCL to the GCB or nonGCB subgroup, frequently provided in routine pathology reports throughout the world, should not be viewed as a reliable prognostic marker by treating physicians. This stated, we would strongly advise caution to use the Hans classifier as the basis for treatment decisions now.
Has the biologic distinction between the GCB and ABC DLBCL subgroups become obsolete in the R-CHOP treatment era? Clearly not, because a huge amount of molecular data, including genetic alterations34 and somatic mutations of several oncogenes affecting the nuclear factor-κB pathway, such as CARD1135 and A20,36 suggest fundamental molecular differences between both DLBCL subgroups that will influence the development of more targeted and risk-adapted therapies.
The emergence of IB morphology as an important and robust risk factor in the RICOVER-60 trial merits special attention. The IB differentiation of DLBCL tumor cells had been described by Lennert a long time ago11 and the negative prognostic impact of IB morphology in DLBCL has been recognized as early as 199730 and, in 2002, data emerging from the Non-Hodgkin Lymphoma Classification Project confirmed the prognostic role of the presence or absence of immunoblasts in DLBCL.31 In addition, IB morphology in DLBCL tumors treated in the CHOP era was associated with inferior outcome in 2 major studies.22,29 Our present work shows that IB morphology is a major risk factor also in DLBCL patients treated with R-CHOP, and that the diagnosis of IB can reproducibly be rendered by hematopathologists. Therefore, this crucial morphologic feature appears to capture at least some of the adverse molecular events that are currently hard to detect with other methods in a routine diagnostic setting.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Karin Schroedl, Steffi Groh, Christine Rudolph, and Inge Klier (Würzburg); Olivera Batic and Christiane Stange (Kiel); Michaela Buck (Ulm); Erika Berg (Berlin); Katharina Vogel und Ilona Schliephake (Lübeck); Ralf Lieberz (Frankfurt); Daniela Rauh (Stuttgart); and Beate Mann (Leipzig) for expert tech-ical assistance.
This work was supported by the Deutsche Krebshilfe (Network Project Molecular Mechanisms in Malignant Lymphoma, Bonn, Germany, grant 70-3173-Tr3).
We dedicate this work to Professor Karl Lennert, whose morphologic principles are still an integral part of lymphoma diagnosis and classification.
Authorship
Contribution: G.O., M.Z., M.L., and A.R. conceived and designed the study; G.O., W.K., A.C.F., H.S., H.-K.M.-H., M.-L.H., P.M., and A.R. provided the pathology reference panel; G.O., W.K., A.C.F., H.S., H.-K.M.-H., M.-L.H., P.M., S.C., M.P., N.S., L.T., and A.R. provided study materials or patients; G.O., M.Z., W.K., M.S., H.H., H.-W.B., C.T., A.C.F., D.L., M.H., H.S., H.-K.M.-H., M.F., M.-L.H., T.F.E.B., P.M., S.C., M.P., N.S., L.T., M.L., and A.R. collected and assembled data; M.Z. and M.L. completed biometry; and G.O., M.Z., M.L., P.M., and A.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: German Ott, Institute of Pathology, Robert-Bosch-Krankenhaus, Auerbachstrasse 110, 70376 Stuttgart, Germany; e-mail: german.ott@rbk.de.