Abstract
Intravenous immunoglobulin (IVIg) is an effective treatment against immune thrombocytopenia (ITP). Previous studies suggested that IVIg exerts this ameliorative role through 2 different leukocyte subsets. Dendritic cells (DCs) modulate the immunosuppression in an adoptive cell transfer model, and phagocytes up-regulate their inhibitory IgG Fc receptors (FcγR)IIB expression and thereby ameliorate the inflammatory response and platelet clearance. However, whether or not regulatory mechanisms exist among DCs, phagocytes, and platelets is still largely unknown. In this study we present findings that IVIg-primed splenic CD11c+ DCs (IVIg-DCs) primarily mediate their anti-inflammatory effects at the level of the platelet rather than the phagocyte. IVIg-DCs did not ameliorate ITP in Fcgr2b−/−, Fcgr3−/−, nor P-Selp−/− mice, implicating the potential involvement of these pathways in IVIg action. As platelets are a component of DC regulatory circuits, these findings may suggest an alternative perspective for the use of IVIg treatment.
Introduction
Immune thrombocytopenia (ITP) is a disorder manifested by immune-mediated low platelet (PLT) counts.1-3 Treatment with high-dose intravenous immunoglobulin (IVIg), a human IgG fraction prepared from pools of plasma of thousands of donors, can rapidly relieve the symptoms of thrombocytopenia.2,4 The PLT clearance is mainly mediated through IgG Fc receptors (FcγR) bearing macrophages in the mononuclear phagocytic system (MPS).5 In mice, both inhibitory and activating FcγRs, the FcγRIIB and FcγRIII (encoded by Fcgr2b and Fcgr3, respectively) are important for IVIg-mediated amelioration.6-8 IVIg treatment was shown to induce macrophages expressing FcγRIIB, by which it reduced the phagocytic activity of the macrophages.5,7,8 In addition, FcγRIII on CD11c+ dendritic cells (DCs) is important for IVIg-mediated amelioration of ITP in a 2-step priming model, in which IVIg could modulate effector leukocytes indirectly through initiator DCs.5,9 The modulation of PLTs, however, is less characterized. The splenic MPS plays important roles in the phagocytic clearance of PLT during ITP,1,10,11 suggesting that the severity of ITP is associated with PLT-phagocyte engagements. Thus, we established a flow cytometry-based PLT-splenic CD14+ leukocyte (PLT-CD14+ LC) engagement analysis to investigate the roles of PLTs and phagocytes in IVIg-primed DCs (IVIg-DCs)-mediated modulations. The involvements of P-selectin, FcγRIIB, and FcγRIII in the amelioration of ITP are discussed.
Methods
IVIg and mice
IVIg was purchased from Bayer (Gamimune N) and CSL Limited Inc. The C57BL/6J mice (males, 7-10 weeks old) were purchased from the National Laboratory Animal Center (NLAC). C57BL/6J mice deficient in FcγRIII (B6;129P2-Fcgr3tm1Sjv/J), FcγRIIB (B6;129S4-Fcgr2btm1Rav/J), and P-selectin (B6;129S2-Selptm1Hyn/J), were purchased from The Jackson Laboratory. Mice were housed in the Laboratory Animal Center of Tzu Chi University. The research methods were approved by the Animal Care and Use Committee of Tzu-Chi University.
Induction and reversal of murine ITP
ITP was induced and treated as previously described.7 Mice were intravenously injected with 0.1 mg/kg body weight of anti-PLT monoclonal antibody (mAb, rat anti-mouse integrin αIIb/CD41 Ig, clone MWReg30; BD Biosciences) to induce ITP in all experiments, except for the low-dose treatments of MWReg30 (0.03 mg/kg) described in supplemental Figure 1 (available on the Blood web site; see the Supplemental Materials link at the top of the online article). To analyze PLT counts, whole blood samples (50-100 μL) of mice were collected from the retro-orbital venous plexus and mixed with anticoagulant ACD solution (38mM citric acid, 75mM sodium citrate, 100mM dextrose) in Eppendorf tubes. PLT counts were then measured with a hematology analyzer (KX-21N; Sysmex) at various time intervals (0, 2, 4, and 24 hours after anti-CD41 Ig MWReg30 treatments). To investigate the ameliorative effect of IVIg on ITP, the mice were intravenously treated with IVIg (1 g/kg) or equivalent vehicle (saline with 100mM maltose, which was used as a stabilizer for IVIg by manufacturer Bayer) 50 minutes before ITP induction (MWReg30 treatments; Figure 1A-D).
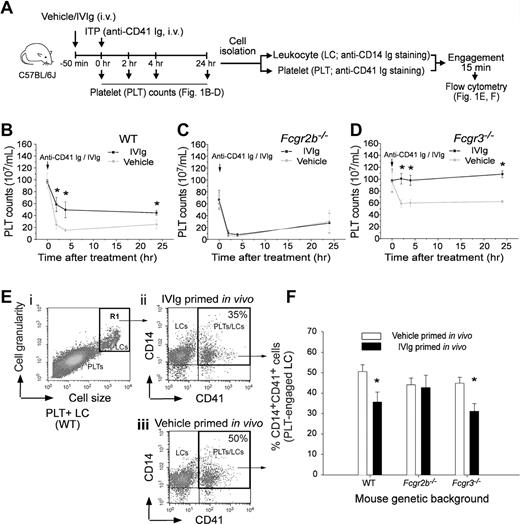
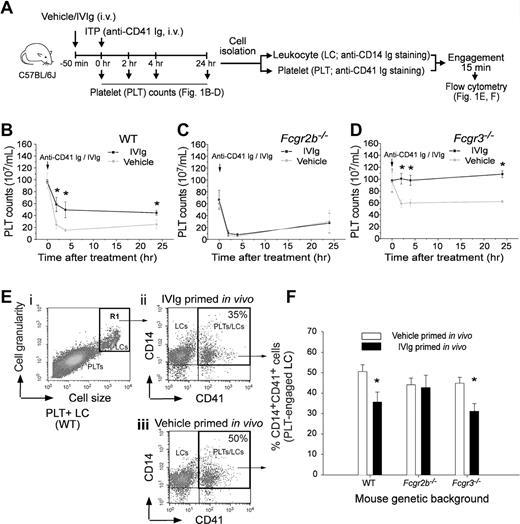
IVIg ameliorated ITP associated with ameliorated platelet-leukocyte engagement. IVIg (1 g/kg) was pretreated 50 minutes to ameliorate anti-CD41 Ig (rat anti-mouse mAb, MWReg30; 0.1 mg/kg) induced ITP and platelet-CD14+ leukocyte (PLT-CD14+ LC) engagement of C57BL/6J mice. The experiment outline is illustrated in panel A. PLT counts of mice with different genetic backgrounds, WT (B), Fcgr2b−/− (C) and Fcgr3−/− (D), at various time intervals after ITP inductions are indicated. *P < .05 versus vehicle groups. n = 8 (4 experiments with 2 replicates). In vitro engagements of lineage specific (PLT: CD41; LC: CD14) fluorescent Igs labeled PLTs and LCs from respective mice groups (B-D) were analyzed using flow cytometry (E-F). LCs were first distinguished from PLTs using cell size and granularity characteristics (Ei, R1 region), and then PLT-engaged LCs were quantified by measuring CD14+ CD41+ double positive cells (top right regions in panel Eii-iii). Quantified results of PLT-CD14+ LC engagements are indicated (% of double positive cells) (F); *P < .05 versus respective vehicle groups. n = 8 (4 experiments with 2 replicates). Data are mean ± SD.
IVIg ameliorated ITP associated with ameliorated platelet-leukocyte engagement. IVIg (1 g/kg) was pretreated 50 minutes to ameliorate anti-CD41 Ig (rat anti-mouse mAb, MWReg30; 0.1 mg/kg) induced ITP and platelet-CD14+ leukocyte (PLT-CD14+ LC) engagement of C57BL/6J mice. The experiment outline is illustrated in panel A. PLT counts of mice with different genetic backgrounds, WT (B), Fcgr2b−/− (C) and Fcgr3−/− (D), at various time intervals after ITP inductions are indicated. *P < .05 versus vehicle groups. n = 8 (4 experiments with 2 replicates). In vitro engagements of lineage specific (PLT: CD41; LC: CD14) fluorescent Igs labeled PLTs and LCs from respective mice groups (B-D) were analyzed using flow cytometry (E-F). LCs were first distinguished from PLTs using cell size and granularity characteristics (Ei, R1 region), and then PLT-engaged LCs were quantified by measuring CD14+ CD41+ double positive cells (top right regions in panel Eii-iii). Quantified results of PLT-CD14+ LC engagements are indicated (% of double positive cells) (F); *P < .05 versus respective vehicle groups. n = 8 (4 experiments with 2 replicates). Data are mean ± SD.
Preparation of IVIg-primed splenic leukocytes
Isolated mouse spleens were placed in 1.5 mL of Eppendorf tubes with 1 mL of collagenase D (Sigma-Aldrich; 2 mg/mL, in 10mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-NaOH, pH 7.4, 150mM NaCl, 5mM KCl, 1mM MgCl2, 1.8mM CaCl2) solution/spleen. Each spleen was injected with 500 μL of collagenase D solution using a 1-mL syringe and then cut into small pieces with scissors. The spleen pieces were incubated in collagenase D solution at 37°C for 15 minutes. The cells were pipetted up and down gently and filtered through a 70-μm strainer (Falcon; BD Biosciences). Red blood cells (RBCs) were lysed with 3 volumes of lysis buffer (150mM NH4Cl, 10mM KHCO3, 0.1mM EDTA) and the LCs were washed twice with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA; PBS-albumin) by centrifuging at 300g for 10 minutes. After incubation with the vehicle control (saline with 100mM maltose) or 20 mg/mL IVIg at room temperature (25°C) for 30 minutes (leukocytes [LCs]; 1 × 107 cells/mL, Tyrode-albumin buffer: 137mM NaCl; 2.8mM KCl; 2mM MgCl2; 0.33mM NaH2PO4; 5mM dextrose; 0.35% BSA; 10mM HEPES, pH 7.4), the LCs were washed twice in saline. The LCs (1 × 106) were finally resuspended in saline (500 μL) before the intravenous treatment of mice.
Preparation of CD11c+ and CD11c− cells
Mice spleens in collagenase D solution were mechanically disrupted and then incubated at 37°C for 15 minutes, gently pipetted up and down, and washed twice in PBS-albumin by centrifugation at 300g for 10 minutes. RBCs were lysed with 3 volumes of lysis buffer [150mM NH4Cl, 10mM KHCO3, 0.1mM EDTA (ethylenediaminetetraacetic acid)], then washed twice in PBS-albumin-EDTA (2mM EDTA), followed by filtering through a 70-μm strainer. CD11c+ and CD11c– cells were prepared from splenic LCs by magnetic separation using a mouse CD11c positive-selection kit (MACS; Miltenyi Biotec) according to the manufacturer's instructions. Splenic LCs (1 × 108 cells in 500 μL in PBS-albumin containing 2mM EDTA) were incubated with 100 μL of CD11c (N418) MicroBeads for 15 minutes at 4 to 8°C. After washing (PBS-albumin-EDTA), cells were placed in a MACS column for magnetic separation. The CD11c– cells were collected in the flow through fraction. The CD11c+ and CD11c– cells were incubated with 20 mg/mL IVIg or vehicle at room temperature for 30 minutes, and then washed twice with saline buffer. The cells (1 × 105) were then resuspended in saline (500 μL) before the intravenous treatment of mice.
Platelet preparation
Whole blood samples collected from the retro-orbital venous plexus of mice were placed into Eppendorf tubes containing anticoagulant ACD solution, which composed 10% of the final volume. The ACD containing blood (500 μL) was gently mixed with 400 μL of Tyrode-albumin buffer, and then centrifuged at 160g for 5 minutes to remove RBCs and white blood cells (WBCs). The supernatant was collected and centrifuged at 160g for 5 minutes again to remove residual RBCs and WBCs. PLT counts were measured using a hematology analyzer (KX-21N; Sysmex).
Flow cytometric analyses for PLT and CD14+ splenic leukocyte (PLT-CD14+ LC) engagement
Three-color fluorescence flow cytometry (FACSCalibur; BD Biosciences) was used to determine PLT-CD14+ LC engagement, using protocols modified from previously described methods.12,13 Isolation of mouse PLTs and splenic LCs was performed as described in the previous sections. Anti-CD41 Ig (MWReg30; fluorescein isothiocyanate (FITC)–conjugated: without FITC-conjugated Ig = 1:1; final 1 μg/mL; BD Biosciences) was used to opsonize and fluorescently label the PLTs (1.5 × 105 cells in 100 μL of PBS-albumin, 30 minutes at 25°C). Allophycocyanin (APC)–conjugated anti-CD14 Ig (eBioscience; 5 μg/mL) was used to label LCs (1 × 105 cells in 100 μL of PBS-albumin, 30 minutes at 25°C). CD14 is a receptor highly expressed on the surfaces of macrophages and monocytes.14-16 After 2 washes to remove unbound Ig (PBS-albumin, PLTs: 1000g, 10 minutes; LCs: 300g, 10 minutes, 25°C), the fluorescently labeled PLTs and LCs were mixed together at room temperature for 15 minutes with gentle shaking (10 rpm) to allow engagement. The ratio of PLTs to LCs was 3:2 (1.5 × 105 PLTs plus 1 × 105 LCs in total 200 μL of Tyrode-albumin buffer). Flow cytometric analysis was used to determine the percentage of CD41 (PLT marker) and CD14 (LC marker) double-positive cells, and thereby determine the levels of PLT and CD14+ LC engagement (Figure 1E-F).
In vitro engagement of in vivo IVIg-primed PLTs and CD14+ LCs
To investigate the regulation of IVIg on PLT and CD14+ LC in vivo, wild type (WT) mice were intravenously primed with IVIg (1 g/kg) or vehicle for 50 minutes. Subsequently, PLTs and LCs were isolated from the mice and then labeled with lineage specific fluorescent Ig as described. After washing twice (PBS-albumin; PLTs: 1000g, 10 minutes; LCs: 300g, 10 minutes), combinations of PLTs and LCs that were previously primed with either IVIg or vehicle in vivo were incubated together to allow PLT-LC engagements as described. The ratio of PLTs to LCs was 3:2 (1.5 × 105 PLTs plus 1 × 105 LCs in total 200 μL of Tyrode-albumin buffer). The quantified results of cell-engagement are shown (Figure 2A experiment outline, 2C columns 1-4).
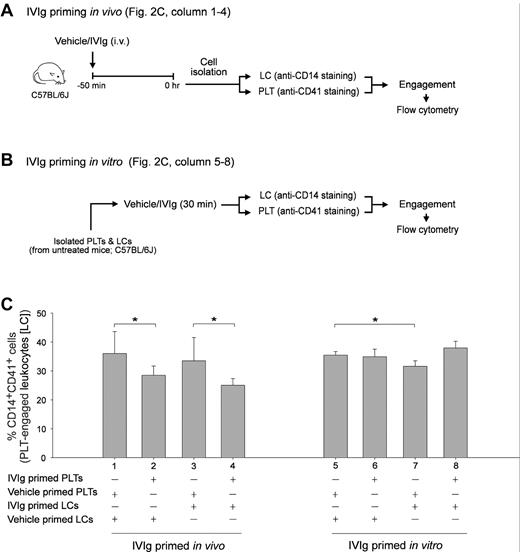
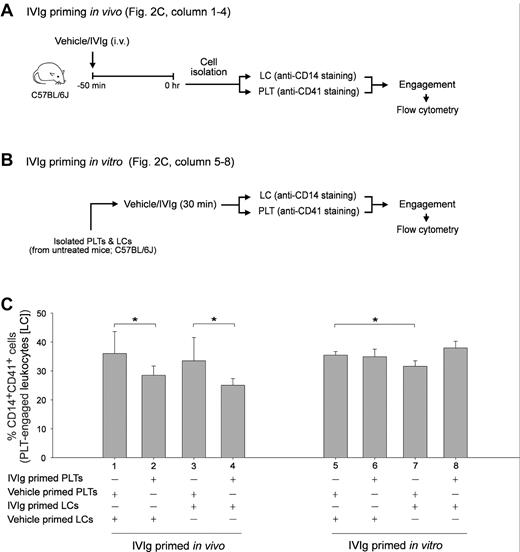
IVIg priming in vivo reduced the cell engagement property of PLTs in vitro. Experimental schemes of in vivo (A) and in vitro (B) IVIg priming of PLTs and splenic LCs and the subsequent PLT-CD14+ LC engagement experiment. Quantitative flow cytometric analysis revealed the relative PLT-CD14+ LC engagement levels as the PLTs and CD14+ LCs were differentially primed with IVIg or vehicle in vivo (columns 1-4) and in vitro (columns 5-8), respectively (C). *P < .05. n = 6 (3 experiments with 2 replicates). Data are mean ± SD.
IVIg priming in vivo reduced the cell engagement property of PLTs in vitro. Experimental schemes of in vivo (A) and in vitro (B) IVIg priming of PLTs and splenic LCs and the subsequent PLT-CD14+ LC engagement experiment. Quantitative flow cytometric analysis revealed the relative PLT-CD14+ LC engagement levels as the PLTs and CD14+ LCs were differentially primed with IVIg or vehicle in vivo (columns 1-4) and in vitro (columns 5-8), respectively (C). *P < .05. n = 6 (3 experiments with 2 replicates). Data are mean ± SD.
In vitro engagement of in vitro IVIg-primed PLTs and CD14+ LCs
To investigate whether IVIg-priming would modulate PLT or LC activity directly, isolated mouse (WT) PLTs or LCs were primed with 20 mg/mL IVIg or vehicle for 30 minutes in Tyrode-albumin buffer. After the priming, PLTs and LCs were subjected to Ig-labeling (PLT: CD41; LC: CD14) and 2 washes. The in vitro PLT-LC engagement and flow cytometric analysis were performed as described. The quantified results of PLT-CD14+ LC engagements are shown (Figure 2B experiment outline, C columns 5-8).
LCs/DCs adoptive transfer and PLT-CD14+ LC engagement
A mouse model was established to investigate whether IVIg-priming could modulate PLT or CD14+ LC activity through splenic LCs or DCs. The protocol used in splenic LC and DC cell-transfer experiments were modified from previously described methods.9 Cells (1 × 106 LCs or 1 × 105 DCs) from the donor mice were intravenously injected into recipient mice after a 30-minute priming with IVIg or vehicle in vitro. Fifty minutes later, these recipients were received MWReg30 treatments (intravenously, 0.1 mg/kg) to induce ITP. Ameliorative effects via adoptive cell-transfer were observed 24 hours after ITP induction by measuring the PLT counts of recipient mice. To determine the extent of PLT-CD14+ LC engagement, anti-CD41 Ig (MWReg30; FITC-conjugated:without FITC-conjugated Ig = 1:1; final 1 μg/mL) was used to opsonize and fluorescently label the PLTs (1.5 × 105 cells in 100 μL of PBS-albumin, 30 minutes at 25°C; applied to both untreated naive and recipient groups). APC-anti-CD14 Ig was used to label the LCs (1 × 105 cells in 100 μL of PBS-albumin, 30 minutes at 25°C; applied to both untreated naive and recipient groups). After fluorescent labeling and 2 washes (PBS-albumin), the PLTs and LCs from the recipient mice were allowed to engage with respective control LC and PLT counterparts (from untreated naive mice) at 25°C for 15 minutes. The levels of PLT-CD14+ LC engagements were analyzed by flow cytometry (Figures 3–4). Naive mice were defined as those without cell transfer. Untreated mice were defined as those without ITP induction and vehicle/IVIg treatments.
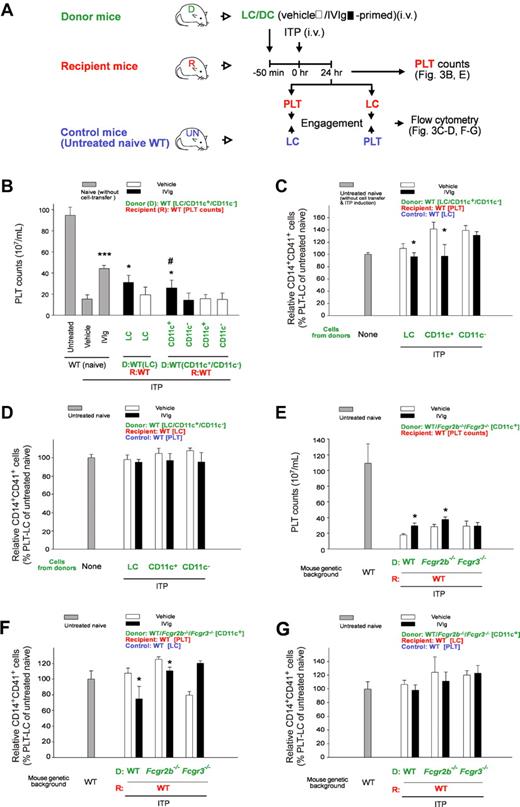
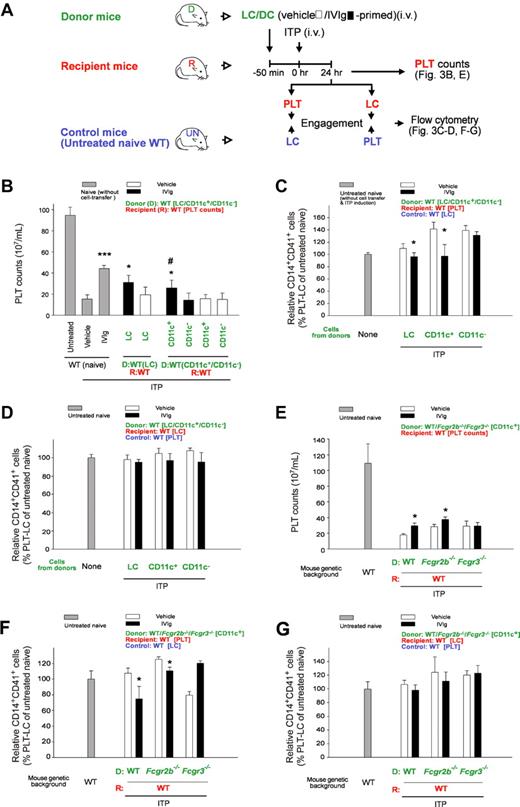
Down-regulated CD14+ LC-engagement property of PLTs is associated with IVIg-DC–mediated amelioration of ITP. Experimental outline (A) for the PLT count (B) and PLT-CD14+ LC engagement (C-D) analyses of recipient mice after adoptive transfer of IVIg-primed splenic cells (LC, CD11c+ DC, and CD11c−) and ITP induction (MWReg30; 0.1 mg/kg). PLTs and LCs were labeled with lineage specific fluorescent Ig (PLT: CD41, LC: CD14) before the engagement. Recipient PLTs and LCs were incubated with respective LC and PLT counterparts from control mice (untreated naive, UN mice; wild type, WT) for 15 minutes and then the percentage of CD14+CD41+ engaged cells were measured by flow cytometry (C-D). *P < .05, ***P < .001 versus respective vehicle groups; #P < .05 versus CD11c− groups. Naive groups n = 10, vehicle and IVIg groups n = 6 (5 and 3 experiments with 2 replicates, respectively). (E-G) As the experiment outline indicated (A), PLT counts (E) and PLT-CD14+ LC engagements (F-G) in the recipient mice were analyzed after the cell-transfer of IVIg-primed splenic CD11c+ DCs (IVIg-DCs; WT, Fcgr2b−/− and Fcgr3−/− donors to WT recipients). (C,F) PLTs from the recipients were engaged with LCs from control mice (UN mice, WT). (D,G) LCs from the recipients were engaged with PLTs from control mice (UN mice, WT). Engagement levels of control PLTs plus control LCs (both from control UN mice, WT) were normalized to 100% (C-D, F-G). *P < .05 represents significant amelioration, versus respective vehicle groups. n = 6 (3 experiments with 2 replicates). D: donor (green labels); R: recipient (red labels); Control: untreated naive mice (blue labels). Data are mean ± SD. Naive: without cell transfer. Untreated: without ITP induction and vehicle/IVIg treatments.
Down-regulated CD14+ LC-engagement property of PLTs is associated with IVIg-DC–mediated amelioration of ITP. Experimental outline (A) for the PLT count (B) and PLT-CD14+ LC engagement (C-D) analyses of recipient mice after adoptive transfer of IVIg-primed splenic cells (LC, CD11c+ DC, and CD11c−) and ITP induction (MWReg30; 0.1 mg/kg). PLTs and LCs were labeled with lineage specific fluorescent Ig (PLT: CD41, LC: CD14) before the engagement. Recipient PLTs and LCs were incubated with respective LC and PLT counterparts from control mice (untreated naive, UN mice; wild type, WT) for 15 minutes and then the percentage of CD14+CD41+ engaged cells were measured by flow cytometry (C-D). *P < .05, ***P < .001 versus respective vehicle groups; #P < .05 versus CD11c− groups. Naive groups n = 10, vehicle and IVIg groups n = 6 (5 and 3 experiments with 2 replicates, respectively). (E-G) As the experiment outline indicated (A), PLT counts (E) and PLT-CD14+ LC engagements (F-G) in the recipient mice were analyzed after the cell-transfer of IVIg-primed splenic CD11c+ DCs (IVIg-DCs; WT, Fcgr2b−/− and Fcgr3−/− donors to WT recipients). (C,F) PLTs from the recipients were engaged with LCs from control mice (UN mice, WT). (D,G) LCs from the recipients were engaged with PLTs from control mice (UN mice, WT). Engagement levels of control PLTs plus control LCs (both from control UN mice, WT) were normalized to 100% (C-D, F-G). *P < .05 represents significant amelioration, versus respective vehicle groups. n = 6 (3 experiments with 2 replicates). D: donor (green labels); R: recipient (red labels); Control: untreated naive mice (blue labels). Data are mean ± SD. Naive: without cell transfer. Untreated: without ITP induction and vehicle/IVIg treatments.
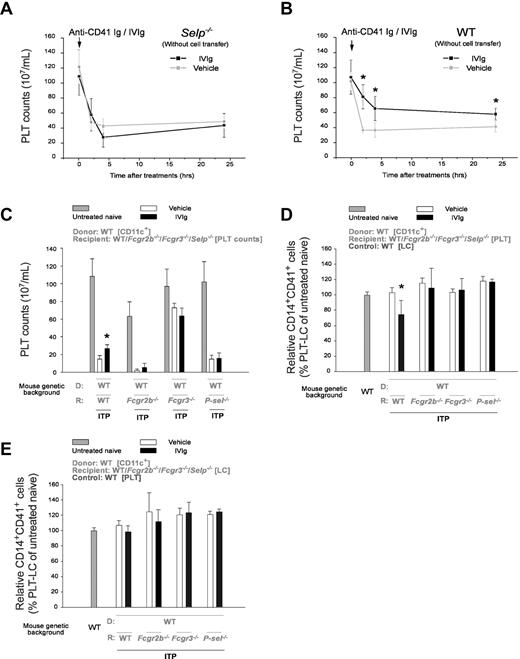
Inability of IVIg-DCs to conduct DC-to-PLT modulation in Selp−/−, Fcgr2b−/−, and Fcgr3−/− mice. (A-D) IVIg treatments could not ameliorate MWReg30-induced ITP in Selp−/− mice (A) compared with WT mice (B). As shown in experiment outline in Figure 3A, after the adoptive transfer of WT vehicle/IVIg-primed DCs and the induction of ITP, the PLT counts of Selp−/−, Fcgr2b−/−, and Fcgr3−/− recipients were analyzed (C). In PLT-CD14+ LC engagements, 2 combinations of recipient PLTs plus control LCs (untreated naive mice, UN-mice) (D), and recipient LCs plus control PLTs (UN-mice; E) were analyzed. PLT-CD14+ LC engagement levels of control PLTs plus control LCs were normalized to 100% (D-E, gray columns). D: donor; R: recipient; Control: untreated naive-mice. *P < .05 represents significant amelioration, versus respective vehicle groups. n = 6 (3 experiments with 2 replicates). Data are mean ± SD. Naive: without cell transfer. Untreated: without ITP induction and vehicle/IVIg treatments.
Inability of IVIg-DCs to conduct DC-to-PLT modulation in Selp−/−, Fcgr2b−/−, and Fcgr3−/− mice. (A-D) IVIg treatments could not ameliorate MWReg30-induced ITP in Selp−/− mice (A) compared with WT mice (B). As shown in experiment outline in Figure 3A, after the adoptive transfer of WT vehicle/IVIg-primed DCs and the induction of ITP, the PLT counts of Selp−/−, Fcgr2b−/−, and Fcgr3−/− recipients were analyzed (C). In PLT-CD14+ LC engagements, 2 combinations of recipient PLTs plus control LCs (untreated naive mice, UN-mice) (D), and recipient LCs plus control PLTs (UN-mice; E) were analyzed. PLT-CD14+ LC engagement levels of control PLTs plus control LCs were normalized to 100% (D-E, gray columns). D: donor; R: recipient; Control: untreated naive-mice. *P < .05 represents significant amelioration, versus respective vehicle groups. n = 6 (3 experiments with 2 replicates). Data are mean ± SD. Naive: without cell transfer. Untreated: without ITP induction and vehicle/IVIg treatments.
Statistical analysis
Statistical differences between groups were calculated using Student t test and were presented as mean ± SD. A P value of < .05 was considered significant.
Results
In this study, we selected an in vitro measurable parameter of PLT-splenic CD14+ LC engagement associated with IVIg amelioration in vivo. Previous study showed that IVIg did not exert an ameliorative role in Fcgr2b−/− mice.7 As expected, we found that IVIg-mediated amelioration of ITP was reproduced in WT (C57BL/6J) and Fcgr3−/− mice (Figure 1A experiment outline; B and D IVIg vs vehicle groups) but not in Fcgr2b−/− mice (Figure 1C anti-CD41 Ig MWReg30, 0.1 mg/kg). To measure PLT-CD14+ LC engagement, purified PLTs and splenic LCs from the same group of mice were first labeled with lineage specific (PLT: CD41; phagocytic LC: CD14) fluorescent Igs and then mixed together for 15 minutes. Subsequently, engaged cells (CD14+ CD41+ double positive) were quantified using flow cytometry (Figure 1 E-F). In agreement with the ameliorative effect on ITP (Figure 1B-D), IVIg treatments significantly reduced PLT-CD14+ LC engagement in WT and Fcgr3−/− mice, but not in Fcgr2b−/− mice (Figure 1F IVIg vs vehicle; *P < .05). As reduced PLT-CD14+ LC engagement is associated with ameliorated ITP, this parameter was used to investigate the influences of IVIg on PLTs and CD14+ LCs, respectively. It revealed that in vivo IVIg priming did not reduce the engagement of CD14+ LCs with control PLTs (vehicle-primed) in vitro (Figure 2A experiment outline C column 1 vs 3), even though the in vitro priming exerted an ameliorative effect on this engagement (Figure 2B experiment outline; C column 5 vs 7; *P < .05). Notably, in vivo but not in vitro IVIg priming of PLTs significantly reduced the engagement regardless of how the CD14+ LCs were treated (Figure 2C column 1 vs 2, 3 vs 4; *P < .05). Contrary to current models focusing mainly on LCs,5,17 these unexpected results imply that IVIg treatments influenced the behavior of PLTs rather than phagocytic LCs in vivo.
CD11c+ DCs are initiator cells in IVIg treatments.9,18 In this study, we combined the LCs/DCs-transfer mouse model9 with our PLT-CD14+ LC engagement analysis to investigate whether DCs regulate PLT function. After being adoptive transfer of in vitro IVIg-primed cells (LCs and DCs) from donor mice, ITP was induced in recipient mice (Figure 3A). To investigate whether PLTs and CD14+ LCs acquired different cell-engagement properties after the cell-transfer, fluorescent Ig–labeled PLTs and CD14+ LCs from the recipients were engaged with their respective (CD14+ LCs and PLTs) control counterparts (from untreated naive mice; UN mice; Figure 3A). In agreement with a previous study,9 we found that IVIg-primed LCs (IVIg-LCs) and CD11c+ DCs (IVIg-DCs), but not CD11c− cells (IVIg-CD11c−s), ameliorated ITP in recipient mice (Figure 3B, IVIg vs vehicle; LC, and CD11c+ vs CD11c−). Again, in accordance with ITP amelioration, PLTs (Figure 3C) but not CD14+ LCs (Figure 3D) from IVIg-LCs and IVIg-DCs transferred recipients, causing considerably lower PLT-CD14+ LC engagements when treated with their respective counterparts from control (untreated naive) mice (Figure 3C vs D; IVIg vs vehicle, LC and CD11c+ vs CD11c− groups; *P < .05). These results suggest that PLTs instead of CD14+ LCs are the primary targets of IVIg-DCs.
Fcgr2b−/− and Fcgr3−/− mice were further used as donors to investigate the involvement of Fcγ receptors in CD11c+ DCs. In agreement with a previous study,9 only IVIg-DCs from WT and Fcgr2b−/− mice but not Fcgr3−/− mice showed ameliorative effects on ITP (Figure 3E). Again, the ameliorative property of IVIg-DCs on ITP (Figure 3E amelioration in WT and Fcgr2b−/−, no effect in Fcgr3−/−) was found similar in PLT-CD14+ LC engagement analysis when the recipients' PLTs (Figure 3F) but not LCs (Figure 3G) were engaged with their respective control counterparts (Figure 3F IVIg vs vehicle, amelioration in WT and Fcgr2b−/−, no effect in Fcgr3−/−; G, no effect in all groups). These results suggest that IVIg modulates PLTs rather than CD14+ LCs through FcγRIII+CD11c+ DCs.
P-selectin (encoded by Selp) is a lineage specific adhesion molecule and an activation marker of PLTs.19 Since P-selectin is responsible for PLT-LC tethering19-21 and plays vital roles in the IVIg-mediated reduction of LC recruitment,22 it is one of the candidate receptors involved in this DC-to-PLT modulation. ITP could be successfully induced in Selp−/− mice, while IVIg failed to exert an ameliorative property compared with the WT control groups (Figure 4A vs B, IVIg vs vehicle). This finding indicates that P-selectin is involved in IVIg-amelioration but not in the induction of ITP. Meanwhile, FcR-mediated destruction of antibody-opsonized PLTs is the primary immune pathophysiology of ITP.23 Thus, Selp−/−, Fcgr2b−/− and Fcgr3−/− mice were used to investigate the necessity of P-selectin and Fcγ receptor expression in the recipients. IVIg-DCs were found to have an ameliorative role in WT mice but not in other mutants (Figure 4C WT recipient vs mutant recipient groups). In agreement with our ITP data, only PLTs from IVIg-DCs transferred WT recipients had a reduced engagement with control LCs (from UN mice) compared with corresponding vehicle groups or other mutant groups (Figure 4D IVIg vs vehicle, WT recipients vs mutant recipients). In contrast, PLT-CD14+ LC engagements were not significantly ameliorated when recipients' LCs and control PLTs were used (Figure 4E WT recipients vs mutant recipients, IVIg vs vehicle). These data suggest that IVIg-DC–mediated suppression of PLT-CD14+ LC engagement requires host expression of P-selectin, FcγRIIB, and FcγRIII.
Discussion
DCs are versatile controllers of the immune system, primarily mediated through interactions with T cells.24 In our PLT-CD14+ LC engagement experiments, however, PLTs instead of CD14+ LCs were the primary targets receiving the suppressive signal from the IVIg-DCs, which distinct from the previously suggested 2-step models.5,17,25 Based on our findings, we propose an alternative hypothesis (supplemental Figure 2). In agreement with a previous study,9 FcγRIII is essential for the initial priming of DCs (Figure 3E-F, no amelioration in Fcgr3−/− groups; supplemental Figure 2A). However, the amelioration of ITP in Fcgr3−/− mice (Figure 1D) suggests that other IVIg-DC independent pathways may exist (supplemental Figure 2B-C; more descriptions of IVIg-DC independent pathways are given below). Meanwhile, FcγRIIB and FcγRIII are important in the downstream regulation of IVIg-DC (Figure 4C-D, no amelioration in Fcgr2−/− and Fcgr3−/−; supplemental Figure 2A1), although their specific roles remain unclear. To stimulate T cells, a maturation process of DCs is required to ensure that the activation of the immune response is coupled to a mechanism capable of discriminating between self and foreign antigens.26 However, whether adoptively transferred IVIg-DCs undergo further maturation in vivo is not clear. As activating and inhibitory FcγRs regulate the threshold of immune responses,27,28 these receptors might be involved in several immune checkpoints before the IVIg-DCs exert their function, and thus may require for recipient mice to support the DC-to-PLT modulation in ITP (supplemental Figure 2A1). Since mouse PLTs do not express FcγRs,29 the requirement for FcγRIIB and FcγRIII in the recipients suggests that other FcγRIIB+ and FcγRIII+ regulatory cells may be involved (supplemental Figure 2A1, RC).
P-selectin is another important molecule to support IVIg-mediated amelioration (supplemental Figure 2A2). In this study, both IVIg and IVIg-DC treatments lacked an ameliorative effect on Selp−/− mice (Figure 4A,C-D Selp−/− groups), suggesting an essential role of P-selectin in the amelioration of ITP. P-selectin is specifically expressed by PLTs and endothelial cells.19 Via tethering to P-selectin ligand 1 (PSGL-1) on LCs, P-selectin mediates the migration of LCs into inflamed tissues through LC-endothelial cell and LC-PLT interactions.21,30 Tissue localization has been shown to greatly affect the function of DCs,31 and P-selectin mediated migration to peripheral tissues was demonstrated to influence the maturation and function of DCs.30,32 In addition, P-selectin/PSGL-1 interaction could positively regulate the generation of tolergenic DCs.33 As a result, P-selectin might be involved in similar regulations for producing functionally active IVIg-DCs. The involvement of PSGL-1 and the functional role of P-selectin in IVIg-DC–mediated amelioration are interesting issues and remain to be further investigated.
We did not observe any obvious regulation of phagocytic CD14+ LCs by IVIg-DCs (Figures 3D,G and 4E). This suggests that IVIg-DC might have only a limited, if any, effect on effector phagocytes in vivo. Up-regulation of inhibitory FcγRIIB on effector LCs was thought to be essential for the anti-inflammatory activity of IVIg.5-8 Paradoxically, in this study, we found that IVIg-DCs transmitted ameliorative signals to PLTs instead of effector LCs. It is possible that IVIg might either directly (supplemental Figure 2B) or indirectly (supplemental Figure 2C) modulate the behavior of effector LCs (ECs), through pathways independent of splenic DC regulation. For example, IVIg priming reduced peripheral LC-endothelial cell interactions in vitro, a process without the involvement of splenic DCs.22 Meanwhile, the level of ITP and the ameliorative effect of IVIg-DCs in the transgenic mice with FcγRIIB-overexpressed macrophages34 should be of some interest. A previous study found that overexpressing FcγRIIB on macrophages seemed insufficient to achieve anti-inflammatory activities compared with IVIg therapy.28 The results we obtained on the IVIg-DC–mediated suppression of PLTs may provide an alternative explanation, since PLT suppressions usually leads to a further reduction in inflammatory responses.35
It is widely accepted that PLTs play important roles in immune responses.36 PLT-to-LC regulations were demonstrated in PLT-mediated inflammatory responses,37-39 and in the modulation of lymphocytes.40,41 Conversely, less attention has been paid to LC-to-PLT modulation. Previous studies were mainly focused on the influence of LC on the downstream immune/defense responses elicited by PLTs,36 while DC-mediated regulation of PLTs in response to engagement of CD14+ LC was not demonstrated. Here we established a feasible assay system to investigate the ameliorative role of IVIg-DCs on ITP. Our results shed light upon the requirement of FcγRIIB, FcγRIII and P-selectin pathways in initiating the downstream responses of IVIg-DCs. Through such regulation, the ameliorative signals are then transmitted from DCs to PLTs. As platelets are downstream targets of the DCs, these findings suggest alternative perspectives for the use of IVIg treatment.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank doctors M. T. Lin and P. Y. Woon for their comments and discussion.
This project was supported by research funding from National Science Council (grant 97WFD2700036) and research funding from Tzu-Chi University (grant TCIRP 98 001/TCIRP 95 002) to H.-H.C.
Authorship
Contribution: H.-H.C. designed the study; H.-S.H. and T.-S.L. performed the experiments; H.-H.C., H.-S.H., and D.-S.S. analyzed and interpreted the data; and H.-H.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hsin-Hou Chang, Department of Molecular Biology and Human Genetics, Tzu-Chi University, Hualien 97004, Taiwan, Republic of China; e-mail: hhchang@mail.tcu.edu.tw.