To the editor:
The combination of lenalidomide and dexamethasone can produce hematologic responses in previously treated patients with AL amyloidosis.1 Since this prospective study (ClinicalTrials.gov: NCT00091260) was initiated, NT-proBNP and BNP have been found to be useful biomarkers for cardiac involvement, prognosis, and response to therapy in patients with AL amyloidosis.2-5 Here we report on the retrospective analysis of the prospectively collected data on changes in BNP during lenalidomide therapy on this trial.
Sixty-eight patients with AL amyloidosis were treated with lenalidomide and dexamethasone at Boston Medical Center. Approval for this study was obtained from the Institutional Review Board of the Boston Medical Center. Informed consent was provided according to the Declaration of Helsinki. The median age was 64 years (range, 42 to 85 years); and 69% were male. Fifty-one patients (75%) had λ clonal plasma cell dyscrasia, and 38 (56%) had cardiac involvement. All patients received lenalidomide and dexamethasone as described in our previous report. Twenty-four of the 68 total patients enrolled did not meet the eligibility to be included in this analysis due to either BNP levels of < 100 pg/mL at baseline and at 1 and 3 months after treatment or unavailability of BNP measurement after 1 or 3 cycles of lenalidomide. Therefore, 44 patients are included in this analysis. An increase in BNP was defined as > 30% increase from baseline value at enrollment on the trial after cycles 1 or 3.
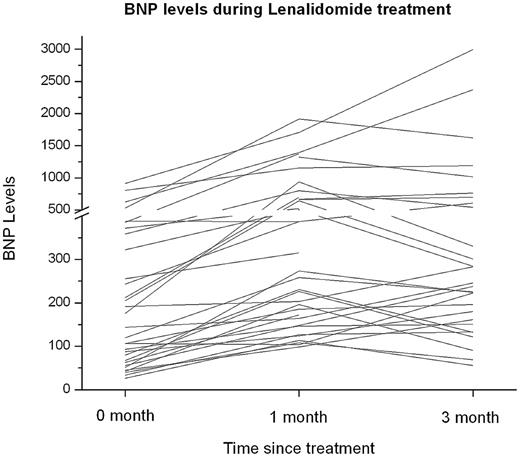
Thirty-eight patients (86%) had > 30% increase in BNP level from baseline, 30 (68%) had an increase after 1 cycle and an additional 8 (18%) patients after 3 cycles of lenalidomide (Figure 1). The mean dose of lenalidomide for patients with an increase in BNP after 1 cycle was 15 mg (range, 5-25 mg) and after 3 cycles was 10 mg (range, 5-15 mg). Of the patients with increase in BNP, only 5 patients (13%) had worsening of renal function by > 50% from baseline. The increase in BNP after 1 and 3 cycles occurred in 23 of 29 patients (79%) with cardiac involvement and 15 of 15 patients (100%) without cardiac involvement. Cardiac troponin I levels were not measured after 1 and 3 cycles of lenalidomide. All the patients with an increase in BNP were asymptomatic without association of modification in NYHA class congestive heart failure.
B-type natriuretic peptide (BNP) levels during treatment with lenalidomide in AL amyloidosis.
B-type natriuretic peptide (BNP) levels during treatment with lenalidomide in AL amyloidosis.
The median survival of these 44 patients is 53 months since initiation of lenalidomide therapy. At these early time points of 1 and 3 months, 20% (n = 9/44) of patients had > 50% improvement in serum free light chain levels, and 2% (n = 1/44) of patients had improvement in BNP of 30% or more.
In conclusion, BNP increased by > 30% in a substantial proportion of patients with AL amyloidosis during treatment with lenalidomide. The mechanism for asymptomatic rise in BNP is not clear; however, the temporal relationship with lenalidomide initiation, the relatively rapid increase, and the absence of other identifiable precipitants for most of the patients suggest that lenalidomide may be playing a role. Moreover, patients with AL amyloidosis receiving lenalidomide whose BNP rises should not be assumed to be failing therapy without other signs of disease progression, but should be monitored closely and treated as needed for signs or symptoms of congestive heart failure should they occur.
Authorship
Acknowledgment: This clinical trial was supported by Celgene Corporation.
Contribution: U.T. designed and performed research, analyzed data, and wrote the manuscript; D.C.S. edited the manuscript; K.T.F. designed research and collected and analyzed data; S.F. and A.S. collected and analyzed data; J.B.Z. designed research and edited the manuscript; and V.S. designed and performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.B.Z. is employed by Celgene Corp, whose product (lenalidomide) was studied in the present work. The remaining authors declare no competing financial interests.
Correspondence: Vaishali Sanchorawala, MD, Section of Hematology/Oncology, FGH 1007, 820 Harrison Ave, Boston, MA 02118; e-mail: Vaishali.Sanchorawala@bmc.org.