Abstract
Hematopoietic stem/progenitor cells (HSPCs) are released from the bone marrow to the circulation by the cytokine, granulocyte colony-stimulating factor, via sympathetic nervous system (SNS)–mediated osteoblast suppression. Because the orientation of HSPCs in their osteoblastic niche is reported to be guided by [Ca2+], we speculated on a cooperation between the calcium-regulating hormones and SNS in the regulation of HSPC trafficking. Here, we present the severe impairment of granulocyte colony-stimulating factor–induced osteoblast suppression and subsequent HSPC mobilization in vitamin D receptor (VDR)–deficient mice. In osteoblasts, functional VDR possessing, at least in part, a transcriptional activity, was specifically induced by β2-adrenergic receptor (AR) agonists. While β2-AR agonists transiently increased mRNA expression of Vdr and its downstream gene, Rankl, 1α,25-dihydroxyvitamin-D3 sustained the β2-AR–induced Rankl expression at high level by stabilizing VDR protein. These data suggest that VDR is essential for durable β2-AR signaling in the stem cell niche. Our study demonstrates not only a novel function of VDR as a critical modulator of HSPC trafficking, but also the presence of a SNS-mediated, bone-remodeling mechanism through VDR. VDR contributes to brain-bone-blood integration in an unanticipated way distinct from other classical calcium-regulating hormones.
Introduction
The great majority of hematopoietic stem/progenitor cells (HSPCs) reside in the bone marrow (BM), particularly in specific niches, such as osteoblasts, that control survival, self-renewal, and proliferation/differentiation.1 It is now well known that the excursion of large numbers of HSPCs from the BM niches to the circulation, which is called mobilization, occurs by the stimulation of a wide variety of agents.2 The cytokine, granulocyte colony-stimulating factor (G-CSF), is most widely used in the clinic for this purpose because it elicits sufficient numbers of HSPCs for life-saving transplantation.
Among accumulating evidences for the regulation of HSPC location, neuronal control of the stem cell niche has emerged very recently.3-5 G-CSF induces the suppression of osteoblasts, which possibly results in the breakdown of functional osteoblastic niches.3,6 Osteocalcin, a sensitive biomarker of mature osteoblasts, is known to be decreased during G-CSF administration.7 Osteopenia has been reported in long-term exposure to G-CSF, such as G-CSF transgenic mice and patients treated chronically with G-CSF due to severe congenital neutropenia.8,9 This G-CSF–induced osteoblast suppression is largely mediated by the sympathetic nervous system (SNS), wherein catecholamines released after G-CSF administration stimulate β2-adrenergic receptors (ARs) on osteoblasts resulting in the suppression of their activity.3 This is consistent with the notion that deficiency in leptin, its receptor, or β2-AR leads to increased bone formation.10,11
The identification of an osteoblastic niche for HSPCs is likely regulated by the level of serum calcium ([Ca2+]) in the endosteal area of the BM. HSPCs in calcium-sensing, receptor-deficient mice can enter the BM cavity after intravenous transplantation, but cannot reach the endosteal region, where [Ca2+] is extremely high in association with bone remodeling.12 Thus, factors that control calcium homeostasis, so-called calcium-regulating hormones (CRHs), such as 1α,25-dihydroxyvitamin-D3 (1,25(OH)2D3), would play important roles for HSPC trafficking in mammals. Although a bioactive vitamin D receptor (VDR) had been demonstrated in normal human blood cells,13,14 it has been reported that the mice lacking VDR (Vdr−/−) display normal numbers of peripheral blood cells and BM progenitors.15 Moreover, BM colony-forming cells of Vdr−/− have been shown to respond normally to several cytokines, including G-CSF in vitro.15
Considering the importance of CRHs in both bone metabolism and HSPC trafficking, we speculated a cooperation between the CRHs and SNS. In search of this, we analyzed Vdr−/− mice in further detail.
Methods
Animals
Vdr−/− mice generated by gene targeting16 were back-crossed more than 9 generations into C57BL/6 background. Vdr+/− breeding pairs produced wild-type (WT) and Vdr−/− littermates, which were used for experiments performed between 6-8 weeks of age. Weanling mice (2-3 weeks old) were fed a normal diet (containing 1.05% calcium and 1.06% phophorus with no lactose) or a high-calcium diet (containing 2.0% calcium and 1.25% phophorus with 20% lactose; Japan Clea).17 We used the high-calcium diet to correct the major phenotype of Vdr−/− mice, rickets type II, represented by growth retardation and hypocalcemia (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). C57BL/6-CD45.1 congenic mice and Caveolin-1−/− mice were purchased from The Jackson Laboratory. All animal experiments were approved by the institutional animal care and use committee and were carried out according to the Kobe University Animal Experimentation Regulations.
Reagents
Isoprenaline and clenbuterol were purchased from Sigma-Aldrich. The mouse osteoblastic cell line, MC3T3-E1, was obtained from RIKEN Cell Bank.
Mobilization of HSPCs
Mobilization of HSPCs by G-CSF was induced as described previously.3 Briefly, the mice were injected with recombinant human G-CSF (Filgrastim; Kyowa Kirin, 250 μg/kg/d, every 12 hours, in 8 divided doses, subcutaneously) in phosphate-buffered saline (PBS), supplemented with 0.1% bovine serum albumin (BSA) and were bled 3 hours after the last dose of G-CSF. Mobilization by AMD3100 (Sigma-Aldrich) was performed as described elsewhere (mice were bled 1 hour after the injection 5 mg/kg, subcutaneously).18
The number of colony-forming units in culture (CFU-C) was assessed as previously described.19 Stem cell activities of blood mobilized by G-CSF were assessed by long-term competitive reconstitution as described previously.19,20 Briefly, 150 μL of mobilized blood was mixed with 2 × 105 BM-nucleated cells (BMNCs) from a CD45.1 competitor donor and were, together, injected into lethally irradiated (14 Gy, 2 split doses) CD45.1-recipient mice. The proportion of peripheral blood leukocytes bearing CD45.1 or CD45.2 antigen was determined monthly after transplantation by flow cytometry. The repopulating units (RUs) were calculated using the following standard formula: RU = % (C)/(100 − %), where % is the measured percentage of donor cells (CD45.2+ cells derived from mobilized blood), and C is the number of competitor marrow cells per 105.
Generation of chimeric mice
Chimeric mice were generated by injection of 2 × 106 WT or Vdr−/− (CD45.2) BMNCs into lethally irradiated (14 Gy, split dose) CD45.1 mice. Reciprocal transplantation was also performed using WT and Vdr−/− mice grown on a high-calcium diet as recipients. Reconstitution by donor cells was confirmed in all mice by blood cell count and CD45.1/CD45.2 chimerism of peripheral blood leukocytes 7-10 weeks after transplantation, and G-CSF-induced mobilization was performed 1 or 2 weeks later.
Flow cytometry
All antibodies and procedures for flow cytometric analysis, including lineage-Sca-1+c-kit+ (LSK) cells and CD45.1/45.2, were performed as previously described.19
Immunofluorescence staining
Fresh-frozen sections of femoral bone were obtained using a tungsten carbide knife and adhesive film (Kawamoto method; Finetec). After the fixation with 4% paraphormaldehyde and blocking with PBS containing both 2% goat and 5% fetal bovine serum (FBS), sections were incubated with rabbit anti–mouse osteocalcin polyclonal antibody (Takara Bio), visualized by Alexa 488–conjugated goat anti–rabbit immunoglobulin G (IgG; Molecular Probes) and mounted in Vectashield Mounting Medium with DAPI (4,6 diamidino-2-phenylindole; Vector Laboratories). Images were captured with a confocal laser scanning microscope (LSM 700; Carl Zeiss). Simultaneous staining with control rabbit IgG showed negligible signals.
ELISA
Enzyme-linked immunosorbent assays (ELISAs) for osteocalcin and CXCL12 were done exactly as described previously.3
Real-time PCR
RNA extraction and quantitative reverse-transcription (RT)–polymerase chain reaction (PCR) were performed as described previously.3 Primers used and PCR conditions are available in supplemental Table 1. All experiments were done in triplicate and normalized to β-actin.
Immunoblotting
Calvarial osteoblasts were obtained from 1-3-day-old mice as described elsewhere.21 Differentiation of MC3T3-E1 cells and primary osteoblasts was induced by culturing in α-minimum essential medium (MEM), supplemented with 10% FBS, 100 μg/mL ascorbic acid, 10mM β-glycerophosphate, and 10−8 M dexamethasone, for 2 weeks. Immunoblot analysis using anti-VDR (C-20; Santa Cruz Biotechnology) and anti–β-actin (Sigma-Aldrich) antibodies was performed as described elsewhere.22
Bone histomorphometry
Bone histomorphometry was performed on extensively ethanol-fixed, undecalcified sections, and parameters were measured at the Ito Bone Histomorphometry Institute (Niigata, Japan).
Transfection and luciferase reporter gene assays
MC3T3-E1 cells were seeded into 6-well tissue-culture plates (12.8 × 105 cells per well) and cultured for 24 hours in α-MEM containing 10% FBS. Cells were transfected with luciferase reporter plasmid DNA containing 2 vitamin D responsive elements (VDREs) of the human 24-hydroxylase gene kindly provided by Dr Hiroshi Eguchi (Teijin Co Ltd)23 using Lipofectamine LTX and PLUS Reagents (Invitrogen). Renilla luciferase plasmid pRL-TK (Promega) was used as an internal control for transfection efficiency. After 4 hours of transfection, the cells were washed once, cultured in α-MEM containing 10% FBS for 10 hours, and then incubated with or without 100μM clenbuterol for 2 hours. The cells were washed and pretreated in the presence of 10−8M 1,25(OH)2D3 for indicated periods. Luciferase activities were determined using a dual-luciferase reporter assay system and a Luminometer 20/20n (Promega).
Statistical analysis
All values were reported as mean ± SEM. Statistical significance for 2 unpaired groups was assessed by the Student t test or Mann-Whitney U test. Significance was set at P < .05.
Results
Hematopoietic parameters in Vdr−/− mice
To reconfirm normal myelopoiesis in the Vdr−/− BM,15 precise assessment of the hematopoietic parameters of Vdr−/− was conducted. Blood-cell counts of Vdr−/− were normal, except for the slight decrease in platelet count (supplemental Table 2). BM cellularity was also slightly decreased in accordance with the growth retardation due to rickets type II16 (supplemental Figure 2A). The frequency of BM CFU-C was comparable with WT littermates (supplemental Figure 2B-C). However, the number of lineage-Sca-1+c-kit+ (LSK) cells was significantly decreased (supplemental Figure 2D-F). This was due to the reduced Sca-1 expression in Vdr−/− because competitive reconstitution assay revealed that the Vdr−/− BM contained normal HSC activity (supplemental Figure 3). Vdr−/− splenic T and B cells showed decreased stem cell antigen 1 (Sca-1) expression even with a high-calcium diet, which corrected the hypocalcemia and the impaired bone formation24 (supplemental Figure 4). In contrast, Sca-1 expression in the Vdr−/− BM was restored with a high-calcium diet (supplemental Figure 5), and competitive reconstitution assay displayed normal stem-cell activity again (supplemental Figure 6). Thus, the number of HSPCs in the Vdr−/− BM was normal.
Impaired G-CSF-induced mobilization in Vdr−/− mice
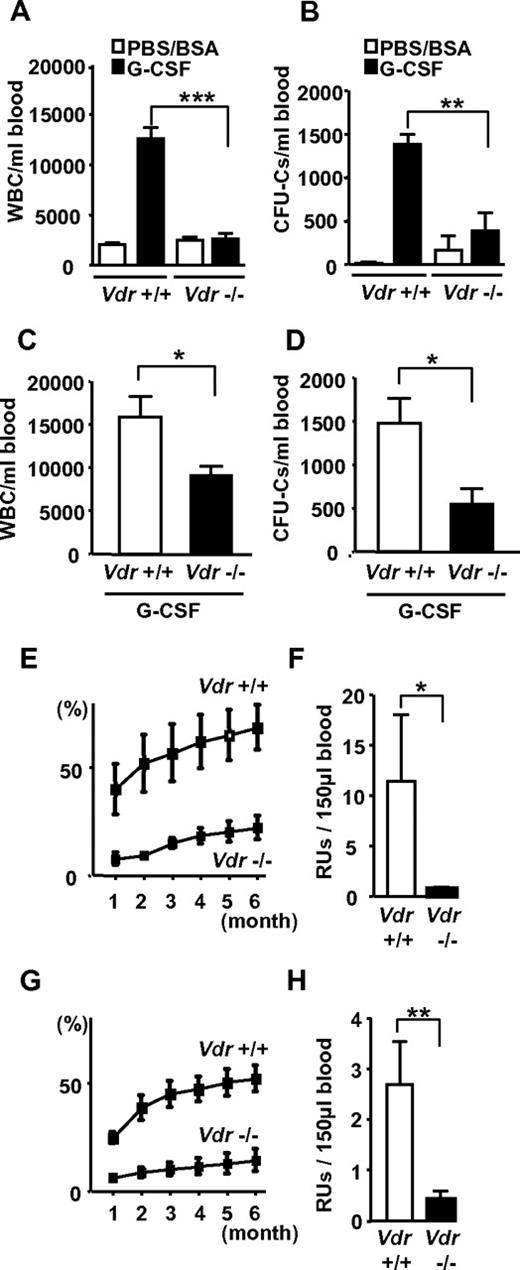
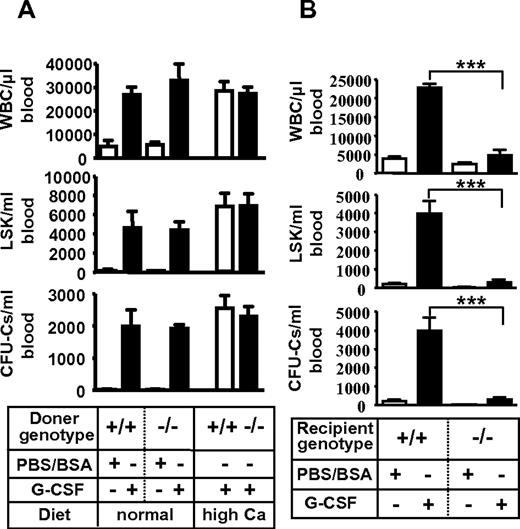
Then, G-CSF–induced HSPC mobilization was examined. Strikingly, G-CSF failed to mobilize mature leukocytes (Figure 1A) and progenitors (Figure 1B) in Vdr−/−. This phenotype of leukocyte number was partially restored in Vdr−/− grown on high calcium diet (Figure 1C), but mobilization of CFU-Cs was still highly suppressed (Figure 1D). A competitive reconstitution assay of mobilized peripheral blood revealed that the mobilization of stem cells (repopulating units: RUs) was also severely impaired (Figure 1E-H). Thus, the HSPCs cannot be mobilized from the BM into the circulation by G-CSF in the absence of VDR.
Impaired G-CSF–induced mobilization in Vdr−/− mice. G-CSF–induced mobilization. Peripheral blood leukocyte count (A) and CFU-Cs (B) in mice fed with a normal diet (n = 4-7), and leukocyte count (C) and CFU-Cs (D) in mice fed with a high-calcium diet (n = 7-9). Percent of CD45.2 in the competitive reconstitution of mobilized blood (E) and calculated RU at 6 months (F) in mice fed with a normal diet (n = 5), and mobilized blood (G) and calculated RU at 6 months (H) in mice fed with a high-calcium diet (n = 6). *P < .05; **P < .01; ***P < .001.
Impaired G-CSF–induced mobilization in Vdr−/− mice. G-CSF–induced mobilization. Peripheral blood leukocyte count (A) and CFU-Cs (B) in mice fed with a normal diet (n = 4-7), and leukocyte count (C) and CFU-Cs (D) in mice fed with a high-calcium diet (n = 7-9). Percent of CD45.2 in the competitive reconstitution of mobilized blood (E) and calculated RU at 6 months (F) in mice fed with a normal diet (n = 5), and mobilized blood (G) and calculated RU at 6 months (H) in mice fed with a high-calcium diet (n = 6). *P < .05; **P < .01; ***P < .001.
Impaired mobilization due to microenvironment insufficiency in Vdr−/− mice
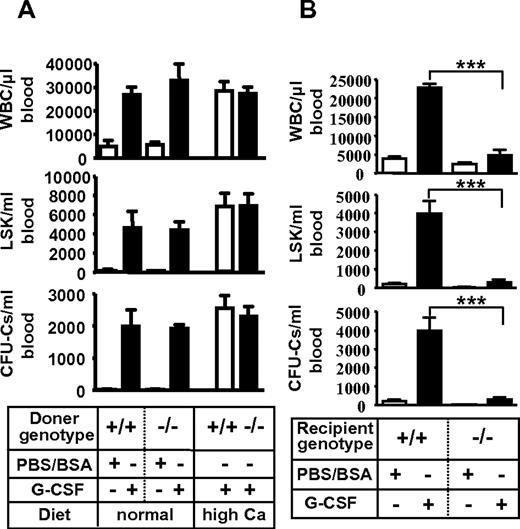
The impaired mobilization was not attributable to VDR deficiency in hematopoietic cells because WT mice reconstituted with Vdr−/− HSPCs, in which progenitor homing and engraftment were normal (supplemental Figure 7; supplemental Table 3), displayed normal mobilization by G-CSF (Figure 2A). It rather seemed to derive from the defect of microenvironment because Vdr−/− transplanted with WT HSPCs, the engraftment of which had been confirmed by blood-cell counts (supplemental Table 3), showed virtually no mobilization by G-CSF (Figure 2B). These results suggest that VDR in hematopoietic microenvironment is essential for G-CSF–induced stem/progenitor mobilization.
Poor mobilization due to microenvironment defect in Vdr−/− mice. (A) Intact mobilization of Vdr−/− hematopoietic cells. CD45.1-congenic wild-type mice reconstituted with Vdr+/+ or Vdr−/− BM were treated with control PBS/BSA (n = 3) or G-CSF (n = 5–6), and the number of circulating CFU-Cs was assessed. (B) Poor mobilization in Vdr−/− microenvironment. Vdr+/+ and Vdr−/− littermates (high-calcium diet) reconstituted with CD45.1 wild-type (WT) BM were treated with control PBS/BSA or G-CSF, and the number of circulating CFU-Cs was assessed (n = 5-6). ***P < .001.
Poor mobilization due to microenvironment defect in Vdr−/− mice. (A) Intact mobilization of Vdr−/− hematopoietic cells. CD45.1-congenic wild-type mice reconstituted with Vdr+/+ or Vdr−/− BM were treated with control PBS/BSA (n = 3) or G-CSF (n = 5–6), and the number of circulating CFU-Cs was assessed. (B) Poor mobilization in Vdr−/− microenvironment. Vdr+/+ and Vdr−/− littermates (high-calcium diet) reconstituted with CD45.1 wild-type (WT) BM were treated with control PBS/BSA or G-CSF, and the number of circulating CFU-Cs was assessed (n = 5-6). ***P < .001.
Impaired mobilization despite decreased CXCL12 in Vdr−/− BM
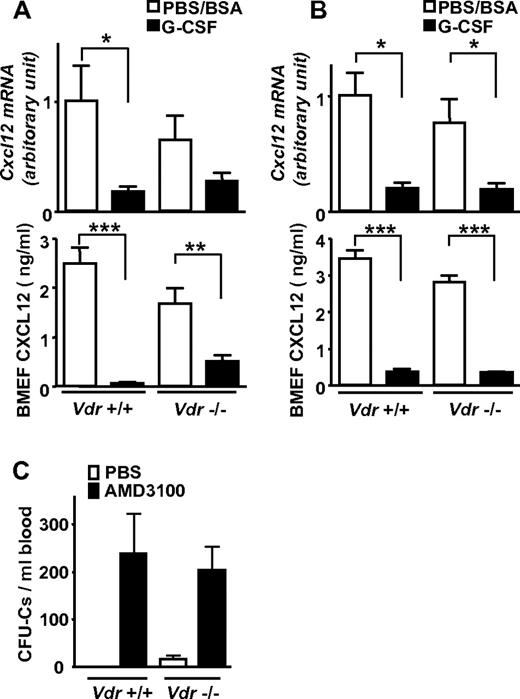
It is well known that the chemokine, chemokine CXCL12 in the BM is reduced in G-CSF–induced mobilization at both protein and mRNA levels. Therefore, it is widely thought that its reduction is a key event for stem-cell release from the BM to the circulation as a consequence of diminished chemoattraction.2,21,25,26 In Vdr−/−, Cxcl12 mRNA was drastically down-regulated in the BM, and CXCL12 in the BM extracellular fluid was also greatly decreased at the protein level after G-CSF administration, despite the absence of mobilization (Figure 3A). These changes were similarly observed even in the mice grown on a high-calcium diet (Figure 3B). Surprisingly, the administration of a CXCR4 antagonist, AMD3100, normally induced mobilization even in Vdr−/− (Figure 3C). Although rapid disruption of CXCL12/CXCR4 axis releases progenitors from the BM in the absence of VDR, the reduction of CXCL12 in the BM is not enough, or may not be the central event, in G-CSF–induced mobilization.
Impaired mobilization despite decreased CXCL12 in Vdr−/− BM. Cxcl12 mRNA in BM and CXCL12 in BM extracellular fluid (BMEF) was drastically decreased, despite highly impaired mobilization in Vdr−/−. (A) mRNA (n = 5-6) and protein (n = 6-9) in mice fed with a normal diet. (B) mRNA (n = 4-5) and protein (n = 4-5) in mice fed with a high-calcium diet. (C) AMD3100-induced mobilization in Vdr−/−. CFU-Cs in mice fed with a normal diet (PBS: n = 3-5; AMD3100: n = 6). *P < .05; **P < .01; ***P < .001.
Impaired mobilization despite decreased CXCL12 in Vdr−/− BM. Cxcl12 mRNA in BM and CXCL12 in BM extracellular fluid (BMEF) was drastically decreased, despite highly impaired mobilization in Vdr−/−. (A) mRNA (n = 5-6) and protein (n = 6-9) in mice fed with a normal diet. (B) mRNA (n = 4-5) and protein (n = 4-5) in mice fed with a high-calcium diet. (C) AMD3100-induced mobilization in Vdr−/−. CFU-Cs in mice fed with a normal diet (PBS: n = 3-5; AMD3100: n = 6). *P < .05; **P < .01; ***P < .001.
VDR is required for G-CSF–induced suppression of mature osteoblasts
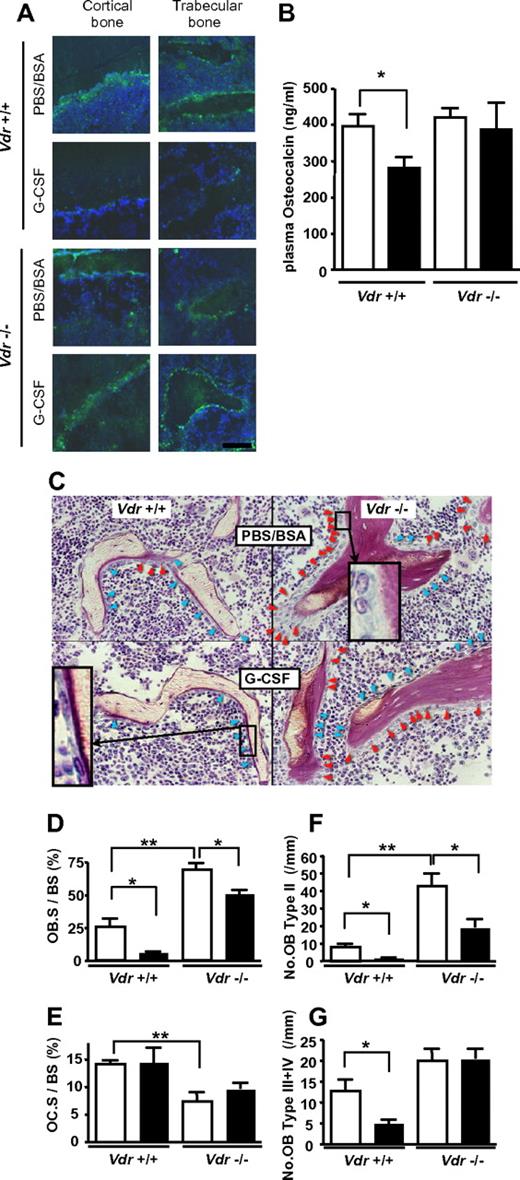
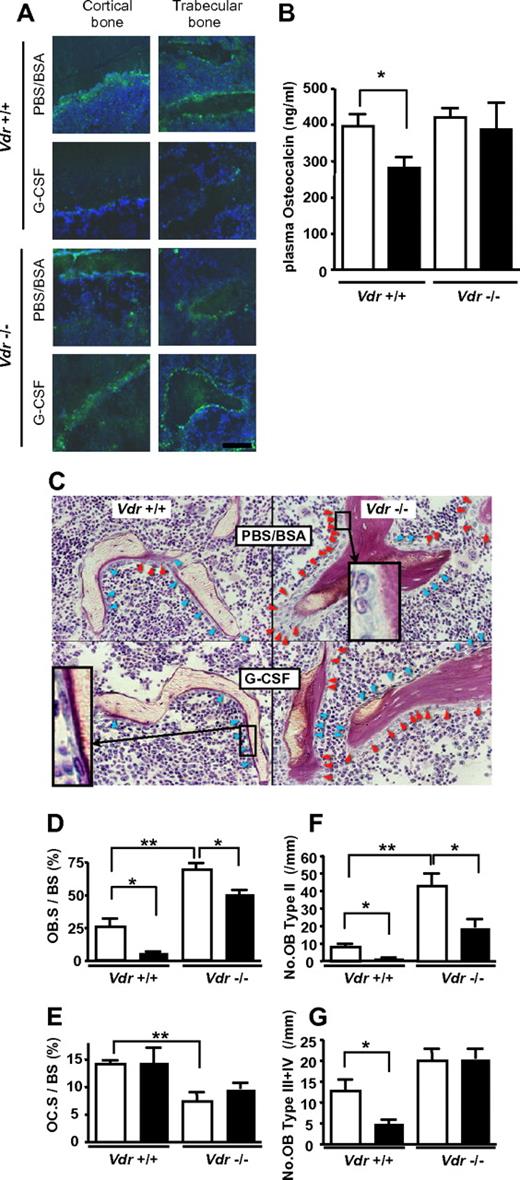
It is also widely known that plasma osteocalcin, a sensitive biomarker of mature osteoblasts, is decreased during G-CSF administration,7 which is consistent with the osteoblast suppression in G-CSF–induced mobilization.3,6 Similar trend was observed by immunofluorescence staining of bone/BM section: as shown in Figure 4A, osteocalcin staining was decreased after G-CSF treatment in WT mice, whereas no decrease was observed in Vdr−/−. This was confirmed by the quantification of plasma level by ELISA. In Vdr−/−, the level of plasma osteocalcin was comparable with that of WT littermates before G-CSF treatment. Consistent with the impaired mobilization, no reduction was observed in G-CSF–treated Vdr−/−, whereas the reduction in WT littermates was evident (Figure 4B). These results suggest that the suppression of mature osteoblast activity by G-CSF did not occur in the absence of VDR. The plasma osteocalcin levels in the littermates grown on a high-calcium diet were also checked. However, it was under the detectable level even in steady-state WT littermates, suggesting that osteocalcin production in osteoblasts is highly suppressed by calcium overload. To further characterize the osteoblasts in association with the alteration of plasma osteocalcin, we performed a histological analysis of the bone. The osteoblast number in Vdr−/− was higher, compared with that of WT, and many osteoblasts were still observed after G-CSF treatment (Figure 4C). Further histomorphological analysis unveiled that the osteoblast surface per bone surface (Ob.S/BS) in WT was reduced by 79% by G-CSF. On the other hand, the reduction was blunted in Vdr−/− (27%), although the baseline was increased (Figure 4D). The osteoclast surface per bone surface (Oc.S/BS) of Vdr−/− was significantly lower than that of WT, but this parameter was not reduced by G-CSF in both WT and Vdr−/− (Figure 4E). We further analyzed the number of osteoblasts carefully according to morphological classification criteria along with the maturational steps as follows: type II: classical cuboidal or columnar with adjacent nuclear clear zone; type III: intermediate without adjacent nuclear clear zone; and type IV: lining transitional cytoplasm—extremely thin, undulating line (most mature population).27 The numbers of osteoblasts in all types were drastically decreased after G-CSF treatment in WT, whereas the number of stretched/spindle-shaped types III and IV, which could, perhaps, include a candidate stem cell niche population,28 remained unchanged in Vdr−/− (Figure 4F-G). Considering the steady-state level of plasma osteocalcin and the morphological change induced by G-CSF, these data suggest that the G-CSF–induced suppression of osteoblastic niche may be cancelled in the absence of VDR.
VDR is necessary for G-CSF–induced suppression of mature osteoblasts. (A) Immunofluorescence staining of osteocalcin. Green: osteocalcin; blue: DAPI. Representative pictures from 2 different sets of experiments are shown. (B) Plasma osteocalcin levels in WT and Vdr−/− were determined by ELISA. n = 5-6. Open bar: PBS/BSA–treated; closed bar: G-CSF–treated. (C) Villanueva stain of trabecular bone of 7-week-old WT and Vdr−/− treated with PBS/BSA or G-CSF. Inset in top right quadrant: typical type II (classical cuboidal or columnar with adjacent nuclear clear zone) osteoblast; bottom left quadrant: typical type IV (lining transitional cytoplasm—extremely thin, undulating line) osteoblast. Red arrowhead: type II osteoblasts; blue arrowhead: type III + IV osteoblasts. (D-G) Histomorphometric indices (n = 4). Osteoblast surface per bone surface (OB.S/BS; D), osteoclast surface per bone surface (OC.S/BS; E), number of type II osteoblasts per mm bone surface (F), and number of type III + IV osteoblast per mm bone surface (G). Open bar: PBS/BSA–treated; closed bar: G-CSF–treated. *P < .05; **P < .01; ***P < .001.
VDR is necessary for G-CSF–induced suppression of mature osteoblasts. (A) Immunofluorescence staining of osteocalcin. Green: osteocalcin; blue: DAPI. Representative pictures from 2 different sets of experiments are shown. (B) Plasma osteocalcin levels in WT and Vdr−/− were determined by ELISA. n = 5-6. Open bar: PBS/BSA–treated; closed bar: G-CSF–treated. (C) Villanueva stain of trabecular bone of 7-week-old WT and Vdr−/− treated with PBS/BSA or G-CSF. Inset in top right quadrant: typical type II (classical cuboidal or columnar with adjacent nuclear clear zone) osteoblast; bottom left quadrant: typical type IV (lining transitional cytoplasm—extremely thin, undulating line) osteoblast. Red arrowhead: type II osteoblasts; blue arrowhead: type III + IV osteoblasts. (D-G) Histomorphometric indices (n = 4). Osteoblast surface per bone surface (OB.S/BS; D), osteoclast surface per bone surface (OC.S/BS; E), number of type II osteoblasts per mm bone surface (F), and number of type III + IV osteoblast per mm bone surface (G). Open bar: PBS/BSA–treated; closed bar: G-CSF–treated. *P < .05; **P < .01; ***P < .001.
Up-regulation of VDR in osteoblasts by β2-adrenergic signals
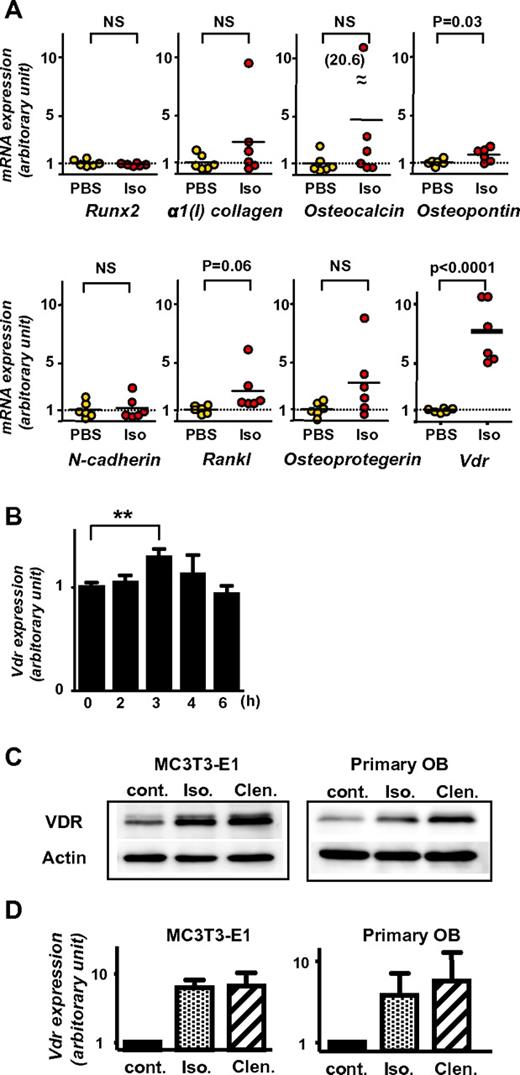
As described in the previous paragraph, VDR is essential for the suppression of mature osteoblasts by G-CSF. Because the suppression is mediated by sympathetic outflow in the BM,3 we speculated that VDR might play an important role in the downstream signaling of β2-AR. To address this, the expression levels of Vdr in the BM before and 2 hours after isoprenaline injection in vivo was first assessed by quantitive RT-PCR. In comparison with several other gene products possibly involved in HSPC trafficking (such as runt-related transcription factor [Runx2], α1(I) collagen, Osteocalcin, Osteopontin, N-cadherin, receptor activator of NF-κB ligand [Rankl], and Osteoprotegerin), Vdr mRNA was drastically up-regulated in all mice after isoprenaline administration. Other transcripts showed no or only slight changes (Figure 5A). The single dose of G-CSF was confirmed to induce a significant increase of Vdr mRNA in the BM in 3 hours and then returned to baseline in 6 hours (Figure 5B).
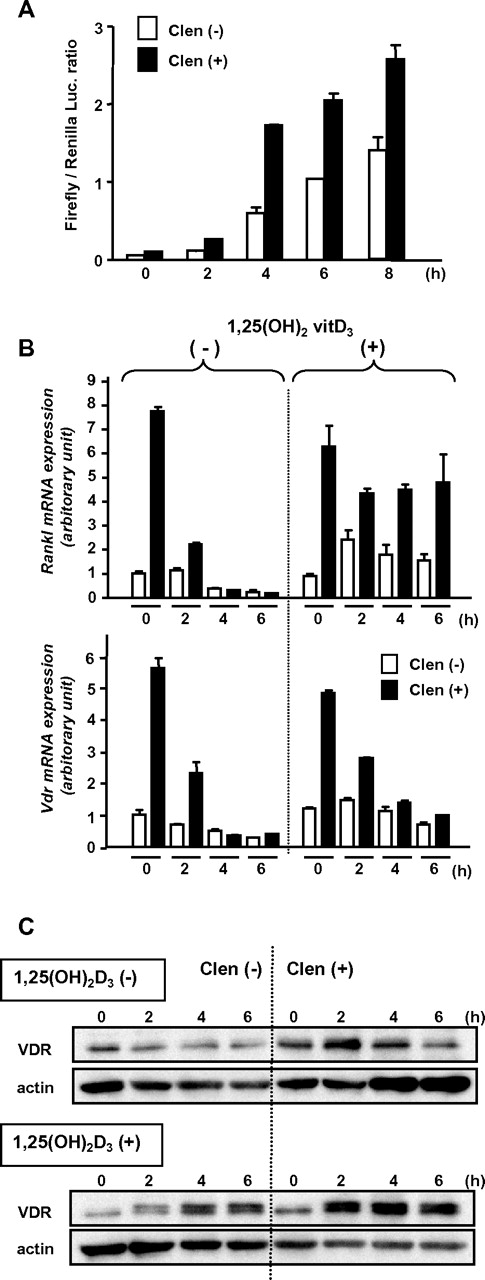
Induction of Vdr expression in BM and osteoblasts by β-AR stimulation. (A) BM was harvested from 7-week-old wild-type C57BL/6 mice 2 hours after PBS or isoprenaline administration (100 mg/kg, intraperitoneal), and expression levels were determined by real-time RT-PCR. Data were normalized to the expression level of β-actin. n = 6. NS, not significant. (B) BM was harvested from 7-week-old wild-type C57BL/6 mice at indicated times after the administration of single dose G-CSF (125 μg/kg, subcutaneously), and Vdr expression was determined by real-time RT-PCR. Data were normalized to β-actin expression. n = 5, 3, 4, 3, and 2 at 0, 2, 3, 4, and 6 hours, respectively. ** P < .01. (C-D) Differentiated MC3T3-E1 and primary carvarial osteoblasts were treated with 100μM pan-β adrenergic receptor agonist, isoprenaline (Iso), or β2-specific agonist, clenbuterol (Clen) for 2 hours, then harvested and processed for immunoblot analysis (C; a representative of 3 independent experiments is shown) and real-time PCR (D; n = 3).
Induction of Vdr expression in BM and osteoblasts by β-AR stimulation. (A) BM was harvested from 7-week-old wild-type C57BL/6 mice 2 hours after PBS or isoprenaline administration (100 mg/kg, intraperitoneal), and expression levels were determined by real-time RT-PCR. Data were normalized to the expression level of β-actin. n = 6. NS, not significant. (B) BM was harvested from 7-week-old wild-type C57BL/6 mice at indicated times after the administration of single dose G-CSF (125 μg/kg, subcutaneously), and Vdr expression was determined by real-time RT-PCR. Data were normalized to β-actin expression. n = 5, 3, 4, 3, and 2 at 0, 2, 3, 4, and 6 hours, respectively. ** P < .01. (C-D) Differentiated MC3T3-E1 and primary carvarial osteoblasts were treated with 100μM pan-β adrenergic receptor agonist, isoprenaline (Iso), or β2-specific agonist, clenbuterol (Clen) for 2 hours, then harvested and processed for immunoblot analysis (C; a representative of 3 independent experiments is shown) and real-time PCR (D; n = 3).
The VDR expression at protein level in osteoblasts treated with β-AR agonists was next evaluated. Short-term (ie, 2 hours) β-adrenergic stimulation in osteoblastic MC3T3-E1 cells and primary calvarial osteoblasts displayed an increased expression of VDR at both mRNA and protein levels (Figure 5C-D). This up-regulation of VDR in mature osteoblasts was likely regulated by β2-AR signaling, because, consistent with the notion that osteoblasts express predominantly this subtype among 3 β-ARs,4,11 the induction was similar or more intense by the β2-specific agonist, clenbuterol, compared with that by the pan-β-AR agonist, isoprenaline (Figure 5C-D).
The function of up-regulated VDR by β2-adrenergic stimulation
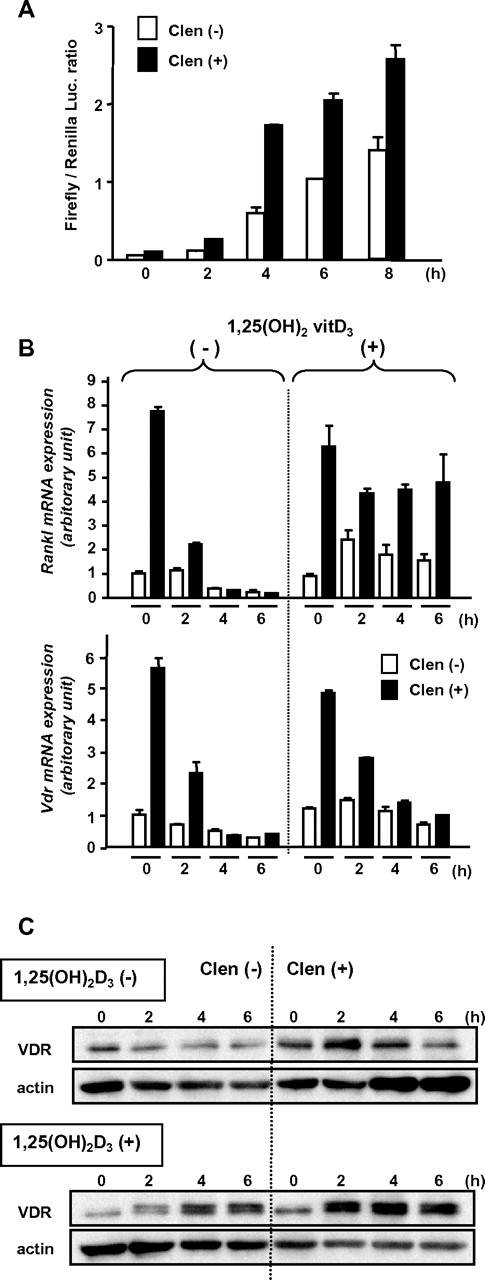
To confirm the functional up-regulation of the β2-AR–induced VDR, we performed a luciferase reporter-gene assay using a plasmid-containing VDRE of the human 24-hydroxylase gene.23 MC3T3-E1 cells transfected with the reporter gene were pretreated with clenbuterol for 2 hours and then cultured in the presence of 1,25(OH)2D3. The enhanced transcriptional activity by the pretreatment indicated that the β2-AR–induced VDR has, at least in part, genomic action (Figure 6A).
Functional up-regulation of VDR induced by β2-AR stimulation. (A) Luciferase reporter gene assay. MC3T3-E1 cells transfected with promoter-reporter plasmid containing VDRE (firefly luciferase) and Renilla luciferase plasmid DNAs were treated with or without clenbuterol for 2 hours and then washed and cultured in the presence of 1,25(OH)2D3 for indicated periods. Each bar shows mean value with error bar of duplicate data. A representative result from 3 independent experiments is shown. (B) Induction of Rankl and Vdr expression. MC3T3-E1 cells were pretreated with clenbuterol for 2 hours and further incubated in the presence or absence of 1,25(OH)2D3 for indicated periods. The expression levels were determined by real-time RT-PCR. Data were normalized to β-actin expression. Each bar shows mean value with error bar of duplicate data. A representative result from 2 independent experiments is shown. (C) Induction and stabilization of VDR by 1,25(OH)2D3. MC3T3-E1 cells were pretreated with clenbuterol for 2 hours and then stimulated by 1,25(OH)2D3 for indicated periods. VDR was detected by immunoblot analysis. A representative result from 3 independent experiments is shown.
Functional up-regulation of VDR induced by β2-AR stimulation. (A) Luciferase reporter gene assay. MC3T3-E1 cells transfected with promoter-reporter plasmid containing VDRE (firefly luciferase) and Renilla luciferase plasmid DNAs were treated with or without clenbuterol for 2 hours and then washed and cultured in the presence of 1,25(OH)2D3 for indicated periods. Each bar shows mean value with error bar of duplicate data. A representative result from 3 independent experiments is shown. (B) Induction of Rankl and Vdr expression. MC3T3-E1 cells were pretreated with clenbuterol for 2 hours and further incubated in the presence or absence of 1,25(OH)2D3 for indicated periods. The expression levels were determined by real-time RT-PCR. Data were normalized to β-actin expression. Each bar shows mean value with error bar of duplicate data. A representative result from 2 independent experiments is shown. (C) Induction and stabilization of VDR by 1,25(OH)2D3. MC3T3-E1 cells were pretreated with clenbuterol for 2 hours and then stimulated by 1,25(OH)2D3 for indicated periods. VDR was detected by immunoblot analysis. A representative result from 3 independent experiments is shown.
It has been reported that RANKL is a mobilization inducer,29 and Rankl expression in osteoblasts is transiently increased by isoprenaline.10 Indeed, Rankl expression was drastically increased in MC3T3-E1 osteoblasts treated with clenbuterol, but rapidly returned to the baseline level in a few hours (Figure 6B). Each expression of Rankl and Vdr induced by clenbuterol was parallel and transient in the absence of 1,25(OH)2D3 (Figure 6B). However, the clenbuterol-induced surge of Rankl expression was maintained for a further period in the presence of 1,25(OH)2D3 (Figure 6B). In contrast, the clenbuterol-induced Vdr expression was rapidly decreased even in the presence of 1,25(OH)2D3 (Figure 6B). In spite of the rapid decrease of Vdr transcript, β2-AR-induced VDR protein was not decreased and was even increased over time in the presence of 1,25(OH)2D3 (Figure 6C). These data suggest that the transient β2-AR stimulation in osteoblasts may increase the stabilized ligand-actived VDR, which keeps the continuous expression of a known downstream gene of VDR, Rankl, even in the absence of intermittent β2-AR stimulation.
Taken together, these in vivo and in vitro data suggest that, after G-CSF administration, β2-AR signals emerging from the peripheral nervous system lead to the production of suppressive signals against HSC niches via functionally up-regulated VDRs, at least in part, by the transcriptional control of its target genes, such as Rankl, in osteoblasts (Figure 7).
Model for the role of VDR in neuronal regulation of hematopoietic stem-cell niche. VDR was up-regulated in osteoblasts by SNS-mediated β2-AR signaling, which was required for the direct suppression of the osteoblastic niche (solid lines), and may also play a role in other niches in the BM parenchyma (dotted lines).
Model for the role of VDR in neuronal regulation of hematopoietic stem-cell niche. VDR was up-regulated in osteoblasts by SNS-mediated β2-AR signaling, which was required for the direct suppression of the osteoblastic niche (solid lines), and may also play a role in other niches in the BM parenchyma (dotted lines).
Discussion
Here, we show that VDR is up-regulated by β2-AR signals, leading to the suppression of osteoblast activity, and that VDR is essential for G-CSF–induced HSPC mobilization. This study has provided with a mechanistic insight that a major CRH receptor, VDR, critically contributes as a pivotal molecule to connect diverse systems (ie, organs) and to compose a regulatory brain-bone-blood triad.30
In a canonical pathway, ligand-activated VDR translocates into the nucleus and subsequently modulates transcription for up- and down-regulation of target genes through the VDRE.31 Considering the drastic, early increase of VDR in osteoblasts after β2-AR stimulation (Figure 5C-D), it is possible that the biological activity of the CRH in osteoblasts may be regulated not only by the plasma concentration of 1,25(OH)2D3, but also by the expression levels of the specific hormone receptor itself. The remarkable up-regulation of VDR by β2-AR signal in osteoblasts may be effective in the induction of rapid, target cell–specific amplification of 1,25(OH)2D3 action. Taken together with a finding that ligand-activated VDR is more stable,32 the neuronal signal-dependent up-regulation of the steroid hormone superfamily receptor suggests a novel cell-specific in situ control mechanism in the microenvironment, in addition to systemic endocrine control of active hormone metabolism.
Steroid hormones can also induce rapid nongenomic responses, which are mediated by the receptors located at the caveolae-enriched membrane fraction.14,31 We wondered whether the caveolar macromolecular signaling domain, including the VDR,33 could play a role in mobilization. However, this turned out to be unlikely, because mobilization by G-CSF was normal in Caveolin-1 knockout mice (supplemental Figure 8).
We found that Rankl expression is latently up-regulated by the transient β2-adrenergic stimulation in combination with VDR signal in osteoblasts (Figure 6B). Kollet et al29 proposed that RANKL-stimulated, bone-resorbing osteoclasts reduced the stem-cell niche components along the endosteum, which was necessary for mobilization. On the other hand, Takamatsu et al7 reported that the inhibition of osteoclast function by bisphosphonate resulted in enhanced mobilization by G-CSF. RANKL seems to be a component of niche control, but its specific role in G-CSF–induced mobilization remains to be elucidated. We also think that many target genes other than Rankl may exist in VDR transcriptional regulation, which would need comprehensive research as a future study.
CXCL12 likely works as an important retention factor in the normal BM, because simple disruption of a CXCL12/CXCR4 pathway by CXCR4 antagonist induces very rapid mobilization.18 It is also the case in the absence of VDR (Figure 3C). After G-CSF treatment, BM microenvironment loses CXCL12 protein through both increased proteolytic activity and decreased CXCL12 mRNA.3,21,26,34 Thus, one might think that CXCL12 down-regulation may be a reasonable explanation for mobilization by G-CSF. However, it is still possible that loss of CXCL12 in the BM during G-CSF treatment represents a coincidental phenotype that does not play a fundamental role in mobilization. It is because highly impaired mobilization in the presence of drastic down-regulation of CXCL12 was already reported in another mouse line, UDP–galactose ceramide galactosyltransferase (cgt)–deficient mice.3 The mice, like Vdr−/−, manifest a problem in the transmission of sympathetic signals in osteoblasts. Two additional mouse models of chemical (6-hydroxydopamine-treated) and genetic (dopamine β-hydroxylase–deficient) sympathectomized mice display no osteoblast suppression in correlation with highly impaired mobilization by G-CSF.3 It is possible that osteoblast suppression by neurotransmitters first leads to the breakdown of HSPC contact with, or reduced production of, niche factors other than CXCL12 from osteoblasts. Thereafter, CXCL12 reduction in the BM originates from osteoblasts2 and/or other cells4,35 and subsequently enhances the mobilization by the guide to the circulation via CXCL12 gradient.
It is reported that parathyroid hormone (PTH) level is high in Vdr−/− mice, which can be corrected only partially by a high-calcium diet, despite a normalized serum calcium level.36,37 Intermittent administration of PTH into normal mice has been reported to target osteoblasts, causing not only an anabolic effect on bone, but also a parallel increase in HSCs.38 In contrast, continuous infusion of PTH is accompanied by osteoclast stimulation and a catabolic effect.39 However, Vdr−/− mice, in which PTH would be continuously high, showed normal HSPC in the BM (supplemental Figures 2,3,5,6), normal plasma osteocalcin level (Figure 4B), and decreased number of osteoclasts (Figure 4E). In addition, it is reported that the administration of PTH induces HPC mobilization.40 It is not clear whether high PTH levels could affect HSPC mobilization efficiency in Vdr−/−.
It seems to be likely that serum calcium level or oral calcium intake affects the hematopoietic system. Although mobilization efficiency of progenitors in normal mice was not altered by a high-calcium diet (Figure 1B-D), there was a trend toward lower stem-cell activity (RUs) in the mobilized blood from normal mice grown on a high-calcium diet (Figure 1F,H; statistically not significant). In addition, HSPCs and lymphocytes in Vdr−/− show a low expression of Sca-1, which was corrected in BM HSPCs, but not in splenic lymphocytes, by a high-calcium diet (supplemental Figures 2,4,5). It might be possible that high calcium intake results in an alteration of calcium concentration at the endosteum by the modulation of bone metabolism. Sca-1 expression in T lymphocytes has been reported to be an activated marker with regulatory functions, such as adhesion, proliferation, and cytokine production.41 It is also suggested to be important for HSPCs in homing into the BM, self-renewal, and interferon-α signaling.41,42 It is of interest whether modification of calcium metabolism by simple oral intake of calcium could be a strategy to regulate stem cell behavior.
Features of niche osteoblasts in the BM were first reported to be spindle-shaped and N-cadherin positive.28 Another study has shown that immature hematopoietic cells are protected from 5-fluorouracil treatment by osteoblasts, which strongly express osteocalcin.43 In this study, it is shown that the decrease in plasma osteocalcin and the number of morphologically mature osteoblasts (ie, stretched/spindle-shaped types III and IV) are well correlated with mobilization efficiency (Figure 4), suggesting that these cells may include a functional niche population. Indeed, among heterogeneous osteoblasts, a fractionated population, which has a higher capability to maintain HSC activity in short-term coculture, expresses higher osteocalcin as well as N-cadherin.44 Thus, osteocalcin-producing mature osteoblasts may contain 1 of the putative niche population, which could be targeted by sympathetic outflow in the presence of VDR after G-CSF administration (Figure 4B,G).
Very recently, nestin-expressing mesenchymal stem cells (MSCs) have been reported to form an HSC niche.45 It would be interesting to examine whether these cells express functional VDR. Also, it would be important to assess the contribution of MSCs or osteoblast precursors as well as fully differentiated osteoblasts in VDR-mediated niche regulation (Figure 7).
In summary, we demonstrate a novel function of the VDR as a critical mediator of neuronal control for the HSPC niche. This implies that modulating agents for VDR may have a potential benefit in controlling HSPC mobilization as well as homing to the BM to improve the clinical efficiency of stem cell transplantation. The exploration of the effective combination of β2-AR agonists and VDR analogs may have a potential benefit not only in neuronal control for the HSPC niche, but also in bone remodeling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr S. Ishizuka for his valuable suggestion.
This work was supported by the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Authorship
Contribution: Y. Kawamori, Y. Katayama, N.A., K.M., and M. Sato performed experiments; A.O., M. Shimoyama, and M.T. provided intellectual input; K.N. and T.O. back-crossed the mice; S.K. generated Vdr−/− mice; and Y. Kawamori, Y. Katayama, and T.M. designed the study, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshimitsu Matsui, Department of Medicine, Kobe University Graduate School of Medicine, 7-5-1 Kusunoki-cho, Chuo-ku, Kobe 650-0017, Japan; e-mail: matsui@med.kobe-u.ac.jp.