Abstract
Naive T cells continuously recirculate between secondary lymphoid tissue via the blood and lymphatic systems, a process that maximizes the chances of an encounter between a T cell and its cognate antigen. This recirculation depends on signals from chemokine receptors, integrins, and the sphingosine-1-phosphate receptor. The authors of previous studies in other cell types have shown that Rac GTPases transduce signals leading to cell migration and adhesion; however, their roles in T cells are unknown. By using both 3-dimensional intravital and in vitro approaches, we show that Rac1- and Rac2-deficient T cells have multiple defects in this recirculation process. Rac-deficient T cells home very inefficiently to lymph nodes and the white pulp of the spleen, show reduced interstitial migration within lymph node parenchyma, and are defective in egress from lymph nodes. These mutant T cells show defective chemokine-induced chemotaxis, chemokinesis, and adhesion to integrin ligands. They have reduced lateral motility on endothelial cells and transmigrate in-efficiently. These multiple defects stem from critical roles for Rac1 and Rac2 in transducing chemokine and sphingosine-1-phosphate receptor 1 signals leading to motility and adhesion.
Introduction
The continuous recirculation of naive T lymphocytes between secondary lymphoid organs (SLOs), such as lymph nodes (LNs) and spleen, through the blood and lymphatic systems is vital for an efficient adaptive immune response.1 T cells in the blood enter LNs through a specialized vasculature termed high endothelial venules (HEVs). Fast-moving T cells in the bloodstream initially interact with HEVs via L-selectin (CD62L) on the surface of the lymphocyte binding to PNAd on endothelial cells, causing the T cells to slow down and roll on the endothelium.1 Subsequently, binding of either the CCL19 or CCL21 chemokines presented on endothelial cells to their receptor CCR7 on T cells results in activation of the integrins lymphocyte function-associated antigen-1 (LFA-1; αLβ2) and VLA-4 (α4β1). These in turn bind to their ligands, intercellular adhesion molecule (ICAM)-1 and ICAM-2 (for LFA-1) and vascular cell adhesion molecule-1 (for VLA-4), on endothelial cells, leading to arrest and firm attachment of the lymphocytes. T cells then migrate laterally on the luminal surface of the endothelial cells before transmigrating through the endothelium, onto its basal side. From here the T cells migrate into the cortical zone of the LN, again under the influence of CCR7-binding chemokines. Intravital microscopy has shown that once within the interstitium of the LN, T cells continue to migrate at high speeds (10-15μm/min) in response to CCR7-binding chemokines.2-7
In contrast to the requirement for integrins in firm adhesion to HEV, these molecules appear to be dispensable for interstitial migration.8 If T cells fail to encounter an antigen-presenting cell with cognate antigen and are not activated, they eventually migrate out of the LN by moving into the medulla, crossing a lymphatic endothelial barrier and entering the efferent lymphatic vessels. From here the T cells are able to migrate through the lymphatic system back into the blood vasculature. This egress from LNs is under the control of sphingosine-1-phosphate (S1P), which binds to the S1P receptor 1 (S1P1) on T cells.9
The Rac GTPases (Rac1, Rac2, and Rac3) are members of the Rho-family of GTPases and have been shown to transduce signals from diverse receptors, leading to cell migration, adhesion, proliferation, and differentiation.10,11 Some studies12,13 have suggested that the Rac GTPases might be involved in the migration, polarization, and adhesion of T cells. Rac2-deficient mouse T cells are partially defective in chemotaxis in response to CCL19, CCL21, and CXCL12 and in homing to LNs. However, the homing defect of Rac2−/− T cells was only observed when the T cells were injected into Rac2−/− mice, making the role of Rac2 within the T cells themselves unclear.12 In human T cells, inhibition of Rac1 interfered with CXCL12-induced adhesion through VLA-4, collapse of microvilli, and inside-out signaling, leading to the switching of LFA-1 into a high-affinity conformation.14-16
Further support for a potential role for Rac GTPases in T-cell recirculation has come from studies of guanine nucleotide exchange factors (GEFs), that activate Rac proteins, several of which are expressed in T cells.17 T cells deficient in DOCK2 and Tiam1 GEFs show defective chemotaxis and homing to LNs, and DOCK2 is required for interstitial migration and egress from LNs.18-22 However, neither GEF is required for transendothelial migration. These defects have been proposed to be a consequence of failure to activate Rac GTPases; however, these GEFs may have GEF-independent functions, as has been shown for Vav1, making the precise roles for Rac GTPases in these processes unclear.23
Despite these aforementioned studies, the potential in vivo functions for Rac GTPases in T-cell migration and adhesion remain largely unknown, and hence they were the focus of the current study. We report for the first time the generation of T cells deficient in both Rac1 and Rac2 and show that the GTPases are critical for T-cell homing to LNs and to the splenic white pulp. We show that Rac-deficient T cells remain less well attached to endothelium, are unable to migrate laterally on the endothelium, and transmigrate much less efficiently through it. We show that Rac GTPases are required for the interstitial mobility of T cells within LNs and for S1P-induced LN egress. Furthermore, we show that these defects are attributable to defective chemotaxis, chemokinesis, and shear-resistant adhesion to integrin ligands.
Methods
Mice
Mice bearing a conditional loxP-flanked Rac1 allele (Rac1flox/flox)24 and Rac2-deficient mice (Rac2−/−)25 were crossed with a transgenic strain carrying the Cre recombinase under the control of the distal Lck promoter (dLck-icre, transgenic line 3779)26 and to reporter mice bearing the R26R-enhanced yellow fluorescent protein (EYFP) allele27 to generate mice deficient in Rac1 (Rac1flox/flox,dLck-icre,R26R-EYFP, referred to as Rac1T), Rac2 (Rac2−/−,dLck-icre,R26R-EYFP, referred to as Rac2−/−), or both (Rac1flox/flox,Rac2−/−,dLck-icre, R26R-EYFP, double-knockout, or DKO). Mice carrying the dLck-icre transgene and R26R-EYFP but no mutations in either Rac1 or Rac2 were used as control wild-type (WT) mice. In all mice analyzed, the dLck-Cre transgene was heterozygous and the R26R-EYFP allele was homozygous.
Flow cytometric analysis and cell sorting
Fluorophore-conjugated antibodies against CD4, CD8, CD44, CD62L, T-cell receptor β (TCRβ), Ly5.1, Ly5.2, and CD11a (αL subunit of LFA-1; BD Biosciences, eBioscience, and Tebu-bio) were used in standard flow cytometric procedures to phenotypically characterize and sort cell populations. To measure levels of F-actin, cells were fixed in 2.5% paraformaldehyde for 10 minutes at room temperature (RT), permeabilized in phosphate-buffered saline (PBS)/0.1% Triton X-100 for 5 minutes at RT, and stained with Alexa Fluor 647–phalloidin (Invitrogen). To measure levels of phospho-Ezrin/Radixin/Moesin (pERM), cells were fixed in 4% paraformaldehyde for 10 minutes at RT, permeabilized in 90% MeOH for 20 minutes on ice, and stained with rabbit anti-pERM (#3141; Cell Signaling), which was revealed with Alexa Fluor 647–conjugated goat anti–rabbit IgG (Invitrogen). Cell sorting was performed on a MoFlo cytometer (Dako), analytical flow cytometry on FACSCalibur, LSRII, and CantoII cytometers (BD Biosciences); data were analyzed by the use of FlowJo Version 9.1 software (TreeStar).
In vitro chemotaxis
LN cells were preincubated in RPMI1640, 1% fatty acid–free bovine serum albumin, 2mM glutamine, and 20mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; chemotaxis medium) for 30 minutes at 37°C 5% CO2. Cells (106) in 100 μL of chemotaxis medium were placed in the upper chamber of 24-well Transwell plates (5-μm pore; Costar), and 600 μL of chemotaxis medium containing no chemokine; CXCL12, CCL19, or CCL21 (200 ng/mL; R&D Systems); or S1P (Sigma-Aldrich) was placed in the lower chamber. Numbers of EYFP+ T cells that had migrated into the lower chamber after 3 hours' incubation were determined by the use of flow cytometry and CaliBrite PerCP beads (BD Biosciences) and were expressed as a percentage of the number of starting T cells, determined from the control wells.
In vivo homing
C57BL/6J mice were injected intravenously with a mixture of 5 × 106 LN cells from one of the 4 dLck-icre mouse strains (all Ly5.2+) and 5 × 106 LN cells from B6.SJL mice (Ly5.1+). The ratio of EYFP+Ly5.2+ to Ly5.1+ T cells (CD4+ or CD8+) in blood, spleen, or LNs was determined 1 hour or 20 hours after transfer by flow cytometry. To determine the anatomical location of transferred T cells, 2.5 × 106 sorted EYFP+ T cells from LNs and spleen of WT or DKO mice were labeled with Cell Tracker Orange CMTMR (5μM; Invitrogen) and carboxyfluorescein succinimidyl ester (CFSE; 5μM) re-spectively, mixed 1:1, and injected intravenously into a C57BL/6J mouse. After 1 hour, the spleen and LNs of the recipient mice were harvested. Parts of these were used for immunohistology, and the rest were analyzed by flow cytometry to determine the ratio of CFSE-labeled to CMTMR-labeled T cells.
Cell adhesion assay
V-bottom 96-well plates (Nunc) were coated overnight with ICAM-1-Fc (3 μg/mL; R&D Systems), blocked with PBS, 2.5% bovine serum albumin, and washed 3 times with PBS. Purified T cells from LNs of WT, Rac1T, Rac2−/−, DKO mice were resuspended at 4 × 106 cells/mL in chemokine medium and aliquoted into the plates at 50 μL/well. Stimulations were started by the addition of 50 μL of medium containing CCL21 (100 ng/mL; final) or MnCl2 (1 mM; final) or no stimulus. The plate was incubated for 1 minute at 37°C followed by 2 successive centrifugations (200g, 2 minutes), incubated for 25 minutes at 37°C, and centrifuged again (200g, 2 minutes). Nonadherent cells accumulated in the bottom of the well, and their EYFP fluorescence was quantitated by the use of a fluorescence plate reader. Percentage adhesion was determined by comparison with wells that had not been coated with ICAM-1, and where no stimulus was added, whose reading was used to determine 0% adhesion.
Egress from LNs
LN cells from WT mice were labeled with CMTMR (2.5μM), mixed with unlabeled LN cells from DKO mice at a 1:1 ratio, and injected intravenously into C57BL/6J mice. In a separate study, the DKO T cells were labeled, and the WT cells remained unlabeled. Twelve hours after lymphocyte transfer, recipient mice received intravenous injections of anti-CD62L (Mel-14; ebioscience, 100 μg/mouse) to prevent further entry of lymphocytes into LNs. Mice were killed 0, 2, and 24 hours after they were injected with antibodies, and flow cytometric analysis was used to assess the rate of egress of injected T lymphocytes from LNs. Transferred T cells were identified as EYFP+, and genotypes distinguished by CMTMR staining. The retention ratio was determined for each time point as the ratio DKO/WT T lymphocytes, normalized to the same ratio calculated at the time of injection of T cells into the mice.
Supplemental methods
For in vitro T-cell interaction with primary mouse brain microvascular endothelial cells, 2-photon intravital microscopy (IVM) of LNs, and video microscopy analysis of T-cell chemokinesis, see the supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical analysis
Statistical comparisons were performed with the use of either the nonparametric 2-tailed Mann-Whitney test or an unpaired 2-tailed t test. Significant differences between mutants and WT (P < .05, P < .01, and P < .001) are indicated in the figures.
Results
Generation of T cells deficient in both Rac1 and Rac2
Mouse T cells, both CD4+ and CD8+, express Rac1 and Rac2, but little or no detectable Rac3 (supplemental Figure 1A).17 Thus, to study the role of Rac GTPases in T-cell migration and homing, we aimed to generate cells missing Rac1, Rac2, or both. Because constitutive deletion of the Rac1 gene results in early embryonic lethality,28 and because Rac1 and Rac2 are required for thymic development of T cells,13,29 we crossed mice bearing a conditional loxP-flanked allele of Rac1 (Rac1flox)24 to a transgenic strain expressing Cre recombinase under the control of the distal Lck promoter (dLck-icre)26 to generate Rac1flox/flox,dLck-icre (hereafter Rac1T) mice and limit deletion of Rac1 to T cells and thymocytes after positive selection. To study the role of Rac2 in T cells, we used mice deficient in Rac2 (Rac2−/−),25 and to generate T cells deficient in both GTPases, Rac1T mice were crossed to Rac2−/− mice to generate Rac1TRac2−/− (double knockout [DKO]) mice. To monitor Cre activity, all these strains were further crossed to mice bearing the R26R-EYFP allele, in which Cre recombinase causes deletion of a transcriptional stop sequence, resulting in expression of EYFP.27 Finally, as a source of control T cells expressing normal levels of Rac1 and Rac2, we used mice bearing only the dLck-icre and R26R-EYFP alleles (WT).
To investigate the efficiency of the dLck-icre transgene and to monitor the effect of loss of Rac1 and Rac2 on T-cell survival, we measured the percentage and absolute numbers of EYFP+ T cells in the blood and SLOs of the 4 different mouse strains. Both percentages and numbers of T cells were reduced in the mutant strains, with the largest reduction observed in DKO mice (supplemental Figure 1B). We note that the numbers of CD8+ T cells were more affected than those of CD4+ T cells. In contrast to SLOs, EYFP+ T cells were less abundant in the blood, and we did not find significant differences in either percentages or numbers between the 4 genotypes. The reduced T-cell numbers in DKO mice are unlikely to be caused by defects in thymic output, because, consistent with previous reports,26 EYFP expression was only seen in the most mature CD4+CD8− and CD4−CD8+ single positive DKO thymocytes, and the numbers of cells in different thymic subsets were unaffected by the mutations (not shown). Thus, our data suggest that Rac1 and Rac2 may contribute to survival of both CD4+ and CD8+ T cells.
Rac1 mRNA levels were greatly reduced in CD4+EYFP+ and CD8+EYFP+ T cells from both LNs and spleen of Rac1T and DKO mice, demonstrating that almost all EYFP+ T cells in these mice had deleted the Rac1 gene (supplemental Figure 1A). The level of Rac3 mRNA was very low in WT T cells and was not significantly increased in DKO T cells, in contrast to readily detectable levels of Rac3 mRNA in mouse hippocampus (supplemental Figure 1A). Hence, we conclude that EYFP+ T cells in DKO mice do not express any Rac GTPases.
Rac1 and Rac2 are required for T-cell homing to SLOs
To investigate the role of Rac GTPases in T-cell homing to SLOs, we examined the localization of mutant T cells in LNs. We found that EYFP+ DKO T cells were located normally throughout the paracortical T-cell zone in LNs, with no evidence of accumulation in or around HEVs (supplemental Figure 2). Despite this normal localization in the steady state, we reasoned that DKO T cells may nonetheless have a reduced ability to home to LNs, which would be more apparent in transient assays.
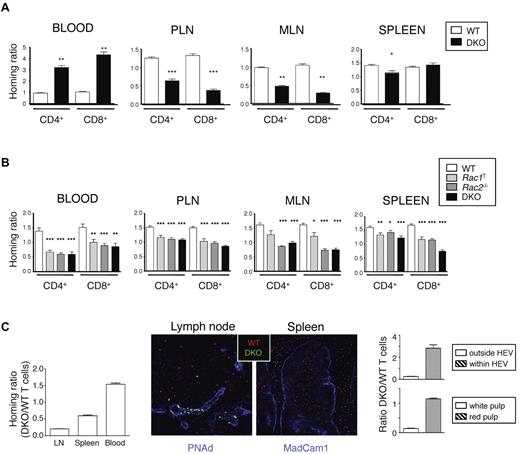
To evaluate this possibility, we transferred WT or mutant T cells into WT mice and measured entry of cells into LNs and spleen. We found that 1 hour after transfer, DKO T cells, both CD4+ and CD8+, were much less efficient at entering both peripheral and mesenteric LNs (PLNs and MLNs); however, there was not much difference in homing to the spleen (Figure 1A). Conversely, many more DKO T cells remained in the blood compared with WT cells, which is consistent with a reduced ability of the DKO T cells to enter LNs. Twenty hours after transfer of mutant cells, DKO T cells were still less efficient at entering PLNs and MLNs compared with WT cells, as were the single mutant Rac1T and Rac2−/− T cells, and Rac-deficient T cells had accumulated less efficiently in the spleen (Figure 1B). Mutant T cells were also underrepresented in the blood 20 hours after transfer, similar to results seen with DOCK2-deficient T cells.18
Rac1 and Rac2 are required for T-cell homing to T-cell areas of LN and spleen. (A, B) LN cells from mice of the indicated genotypes were mixed with LN cells from B6.SJL (Ly5.1+) mice at a 1:1 ratio and transferred intravenously into C57BL/6 recipient mice. Cells were harvested from the blood, PLN, MLN, and spleen either 1 hour (A) or 20 hours (B) after transfer. Graphs show mean ± SEM homing ratio of EYFP+ to Ly5.1+ T cells in the recovered cells normalized to the ratio of EYFP+ to Ly5.1+ T cells in the cells injected into the mice. Data in panel A are from 3 independent experiments (numbers of mice; PLN and spleen: WT, 18; DKO, 20; blood and MLN: WT, 6; DKO, 7). Data in panel B are from 6 independent experiments (numbers of mice: WT, 35; Rac1T, 28; Rac2−/−, 25; DKO, 27). *P < .05; **P < .01; ***P < .001. (C) CMTMR-labeled WT and CFSE-labeled DKO T cells were mixed (1:1 ratio), injected intravenously into C57BL/6 recipient mice (n = 3), and cells harvested from blood, spleen, and LNs 1 hour after transfer. Graph on left shows mean ± SEM homing ratio of DKO to WT T cells in the organs indicated, defined as the ratio of DKO to WT T cells in the recovered cells normalized to the same ratio in the injected cells. Images show immunofluorescence staining of section of spleen or LN from the recipient mice 1 hour after transfer with transferred WT and DKO T cells identified in red and green, respectively. Blue shows staining with anti-PNAd to identify HEV in LNs and anti-Madcam1 in spleen to identify the boundary between red and white pulp. Graph on right shows mean ± SEM ratio of DKO to WT T cells found either within or outside the HEV of LNs and in the red and white pulp of the spleen (15 different areas imaged/organ).
Rac1 and Rac2 are required for T-cell homing to T-cell areas of LN and spleen. (A, B) LN cells from mice of the indicated genotypes were mixed with LN cells from B6.SJL (Ly5.1+) mice at a 1:1 ratio and transferred intravenously into C57BL/6 recipient mice. Cells were harvested from the blood, PLN, MLN, and spleen either 1 hour (A) or 20 hours (B) after transfer. Graphs show mean ± SEM homing ratio of EYFP+ to Ly5.1+ T cells in the recovered cells normalized to the ratio of EYFP+ to Ly5.1+ T cells in the cells injected into the mice. Data in panel A are from 3 independent experiments (numbers of mice; PLN and spleen: WT, 18; DKO, 20; blood and MLN: WT, 6; DKO, 7). Data in panel B are from 6 independent experiments (numbers of mice: WT, 35; Rac1T, 28; Rac2−/−, 25; DKO, 27). *P < .05; **P < .01; ***P < .001. (C) CMTMR-labeled WT and CFSE-labeled DKO T cells were mixed (1:1 ratio), injected intravenously into C57BL/6 recipient mice (n = 3), and cells harvested from blood, spleen, and LNs 1 hour after transfer. Graph on left shows mean ± SEM homing ratio of DKO to WT T cells in the organs indicated, defined as the ratio of DKO to WT T cells in the recovered cells normalized to the same ratio in the injected cells. Images show immunofluorescence staining of section of spleen or LN from the recipient mice 1 hour after transfer with transferred WT and DKO T cells identified in red and green, respectively. Blue shows staining with anti-PNAd to identify HEV in LNs and anti-Madcam1 in spleen to identify the boundary between red and white pulp. Graph on right shows mean ± SEM ratio of DKO to WT T cells found either within or outside the HEV of LNs and in the red and white pulp of the spleen (15 different areas imaged/organ).
Next we examined the location of T cells transferred 1 hour earlier. Although WT T cells had entered the paracortical areas of LNs, DKO T cells were largely localized within or near HEVs (Figure 1C). In the spleen, WT T cells had entered the white pulp, whereas DKO T cells were mainly found in the red pulp. Furthermore, by using 3-dimensional quantitative immunohistology, we found that most WT T cells had entered the LN parenchyma, whereas most DKO T cells were still located in or near HEVs (supplemental Figure 3A-B). Taken together, these studies show that both Rac1 and Rac2 are required for efficient homing of T cells to SLOs with cells arrested at the HEV of LNs and within the red pulp of the spleen. The largest defects were seen in DKO T cells, demonstrating functional redundancy between Rac1 and Rac2.
Rac GTPases are required for chemokine-induced adhesion to, migration on, and transmigration through endothelium
Entry of T cells into LNs requires initial rolling of the cells on HEV through L-selectin/PNAd interactions, followed by chemokine-induced adhesion to integrin ligands, lateral migration on the endothelium, transmigration and, finally, movement away from the basal surface of the endothelium into the parenchyma of the LN. In view of the homing defect of Rac-deficient T cells, we investigated the role of Rac GTPases in these steps by using both in vitro and in vivo assays.
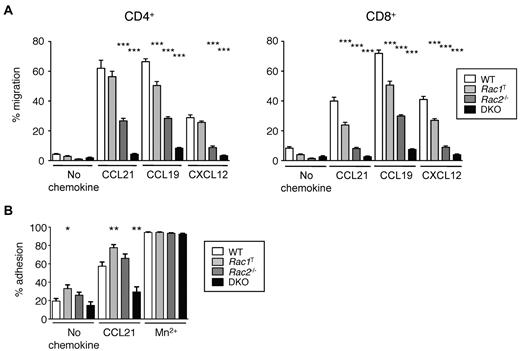
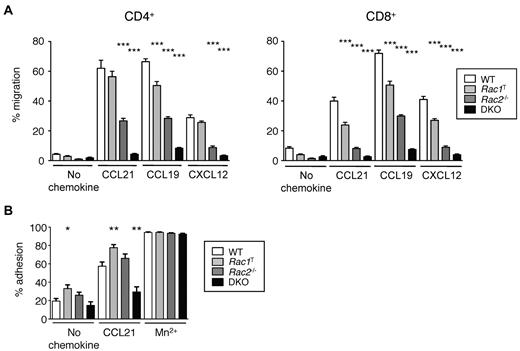
We found that Rac1T T cells, both CD4+ and CD8+, had mild defects in chemotaxis in response to CCL19, CCL21, and CXCL12, Rac2−/− T cells were moderately impaired and DKO T cells were very severely affected (Figure 2A). These defects were not attributable to a lack of CCR7 because Rac-deficient T cells have normal surface levels of the receptor (not shown). These results show that although both Rac1 and Rac2 are required for normal chemokine-induced chemotaxis, with Rac2 playing a more important role, there is clear redundancy of function with both GTPases able to transduce chemokine receptor signals. Furthermore, we found that CCL21-induced adhesion to ICAM-1, the ligand for LFA-1 was reduced in DKO cells, suggesting that part of the homing defect in these cells may be the result of inefficient firm adhesion to HEVs (Figure 2B).
Rac1 and Rac2 are required for chemokine-induced chemotaxis and adhesion of T cells. (A) Graph shows mean ± SEM percentage migration of T cells in a Transwell assay in response to CCL21, CCL19, CXCL12, or in the absence of chemokine. (B) Graph shows mean ± SEM percentage adhesion of T cells of the indicated genotypes to immobilized ICAM-1 in response to CCL21, Mn2+, or no stimulus. Data are from 4 independent experiments. *P < .05; **P < .01; ***P < .001.
Rac1 and Rac2 are required for chemokine-induced chemotaxis and adhesion of T cells. (A) Graph shows mean ± SEM percentage migration of T cells in a Transwell assay in response to CCL21, CCL19, CXCL12, or in the absence of chemokine. (B) Graph shows mean ± SEM percentage adhesion of T cells of the indicated genotypes to immobilized ICAM-1 in response to CCL21, Mn2+, or no stimulus. Data are from 4 independent experiments. *P < .05; **P < .01; ***P < .001.
To evaluate the ability of DKO T cells to adhere to endothelium, migrate upon it, and transmigrate through it, we perfused WT and mutant T cells over an endothelial monolayer prepulsed with CCL21. Initial adhesion of T cells was conducted for 5 minutes at low shear stress, and both WT and DKO T cells adhered normally (not shown). Subsequently, the flow rate was increased to a physiologic level of shear stress. WT T cells were seen to migrate rapidly on top of the endothelial cells, to transmigrate, and to continue rapid migration underneath the endothelium (supplemental Videos 1-2). In contrast, DKO T cells showed profound defects in these processes. Rac-deficient T cells resisted physiologic shear stress less efficiently (Figure 3A), and track analysis showed that DKO T cells migrated much shorter distances both on top of the endothelium and below it (Figure 3B). This was characterized by a reduction in the distance traveled by DKO T cells and their mean velocities (Figure 3C-F). Furthermore, DKO T cells were less efficient at transmigrating through the endothelium (Figure 3G).
Rac GTPases are required for chemokine-induced adhesion to, migration on and transmigration through endothelium. WT and DKO T cells were allowed to attach to an endothelial monolayer at 0.24 dyne/cm2 for 5 minutes, and flow was then increased to 1 dyne/cm2 (high shear stress). Behavior of EYFP+ T cells was recorded by video microscopy. (A) Graph shows mean ± SEM percentage of EYFP+ T cells remaining attached on top of the endothelium after 5 minutes of high shear stress (either crawling or stationary) as a fraction of EYFP+ T cells at the start of the high shear stress period. (B) Flower plots show migration tracks of individual T cells of the indicated genotype, either on top of or below the endothelium, with the starting point of each track placed at the origin (0, 0). Cells that left the field of view during recording were not included. Note that in DKO plots there 44 and 39 cell tracks shown on top and below endothelium, respectively, but most are not clearly visible as they remain close to the origin. (C) Scatter plot of distance traveled by T cells on top of the endothelium. (D) Scatter plot of mean velocities of individual T cells on top of the endothelium. (E) Scatter plot of distance traveled by T cells below the endothelium. (F) Scatter plot of mean velocities of T cells below the endothelium. (G) Graph showing mean ± SEM percentage of T cells that had transmigrated through the endothelium by the end of the video recording (up to 55 minutes), as a fraction of EYFP+ T cells at the start of the high shear stress period. In panels C-F, red lines indicate means. Data are from 5 videos analyzed from 3 independent experiments. *P < .01; ***P < .001.
Rac GTPases are required for chemokine-induced adhesion to, migration on and transmigration through endothelium. WT and DKO T cells were allowed to attach to an endothelial monolayer at 0.24 dyne/cm2 for 5 minutes, and flow was then increased to 1 dyne/cm2 (high shear stress). Behavior of EYFP+ T cells was recorded by video microscopy. (A) Graph shows mean ± SEM percentage of EYFP+ T cells remaining attached on top of the endothelium after 5 minutes of high shear stress (either crawling or stationary) as a fraction of EYFP+ T cells at the start of the high shear stress period. (B) Flower plots show migration tracks of individual T cells of the indicated genotype, either on top of or below the endothelium, with the starting point of each track placed at the origin (0, 0). Cells that left the field of view during recording were not included. Note that in DKO plots there 44 and 39 cell tracks shown on top and below endothelium, respectively, but most are not clearly visible as they remain close to the origin. (C) Scatter plot of distance traveled by T cells on top of the endothelium. (D) Scatter plot of mean velocities of individual T cells on top of the endothelium. (E) Scatter plot of distance traveled by T cells below the endothelium. (F) Scatter plot of mean velocities of T cells below the endothelium. (G) Graph showing mean ± SEM percentage of T cells that had transmigrated through the endothelium by the end of the video recording (up to 55 minutes), as a fraction of EYFP+ T cells at the start of the high shear stress period. In panels C-F, red lines indicate means. Data are from 5 videos analyzed from 3 independent experiments. *P < .01; ***P < .001.
To extend these studies into an in vivo setting, we used 2-photon IVM to image entry of T cells into intact LNs in the living mouse. Examination of videos showed that while WT T cells transmigrated across HEV and moved away from the basal side of the endothelium into the interstitial areas of the LN, DKO cells were relatively immobile and remained in close contact with the HEV, although some DKO cells were able to transmigrate (supplemental Videos 3-4). Taken together, we conclude that Rac1 and Rac2 are required in T cells for shear-resistant adhesion to HEV, lateral migration on the luminal surface of the endothelium, transmigration, and locomotion away from the basal surface of the endothelium into LN interstitium. A combination of defects in these processes is likely to account for the inefficient homing of DKO T cells to LNs.
Rac1 and Rac2 are required for chemokinesis and for interstitial movement within the LN
Although our analysis showed that Rac1 and Rac2 are required for efficient entry of T cells into LNs, Rac-deficient T cells are not completely blocked in this process and eventually accumulate in significant numbers within SLOs (supplemental Figure 2 and Figure 1B). Thus, we were able to examine the role of the GTPases in interstitial motility within the LN paracortex. Using 2-photon IVM, we monitored the motility of WT or mutant T cells transferred 15-72 hours earlier (supplemental Videos 5-7). This analysis demonstrated that Rac1T T cells had an unaltered interstitial mobility compared with WT T cells, with a mean velocity of approximately 15 μm/min, whereas Rac2−/− T cells had a slightly reduced motility of approximately 12 μm/min (Figure 4A, Table 1).6,30 In contrast, DKO T cells showed a very large reduction in interstitial mobility compared with WT T cells, with mean and instantaneous velocities reduced to 5.6 and 4.2 μm/min, respectively (Figure 4A, Table 1). This finding was associated with a large decrease in the motility coefficient and meandering index of the DKO cells and an increase in their turning angles compared with WT T cells (Figure 4B-D, Table 1). These results show that Rac1 and Rac2 are essential for normal interstitial motility and that there is considerable functional redundancy between Rac1 and Rac2 in this process.
Interstitial movement of T cells within LNs. Purified WT EYFP+ T cells and EYFP+ T cells from Rac1T, Rac2−/−, or DKO mice were labeled with CFSE or CMTMR, mixed, and transferred into C57BL/6 recipient mice. Interstitial movement of labeled T cells within the popliteal LN was analyzed 15-72 hours later by multiphoton intravital video microscopy. All results are presented as a pair-wise comparison of WT with Rac1T, Rac2−/−, or DKO T cells. (A) Scatter plot of mean velocities of individual T cells. Red bar indicates the median of these velocities. (B) Graphs show the mean displacement of T cells as a function of √(time). The slope of this graph was used to determine the motility coefficient, a measure of the ability of a cell to move away from its starting position (Table 1).6,30 (C) Turning angles of individual T cells measured as the change in direction of movement occurring between successive frames. (D) Top, a cumulative distribution plot of the meandering index (displacement/path length) of T cells of the indicated genotypes. Bottom, the median meandering index (± interquartile range). The meandering index is a measure of the straightness of track. WT v Rac1T: 13 videos analyzed from 6 independent experiments; WT v Rac2−/−: 14 videos analyzed from 9 independent experiments; WT v DKO: 7 videos analyzed from 4 independent experiments. *P < .001.
Interstitial movement of T cells within LNs. Purified WT EYFP+ T cells and EYFP+ T cells from Rac1T, Rac2−/−, or DKO mice were labeled with CFSE or CMTMR, mixed, and transferred into C57BL/6 recipient mice. Interstitial movement of labeled T cells within the popliteal LN was analyzed 15-72 hours later by multiphoton intravital video microscopy. All results are presented as a pair-wise comparison of WT with Rac1T, Rac2−/−, or DKO T cells. (A) Scatter plot of mean velocities of individual T cells. Red bar indicates the median of these velocities. (B) Graphs show the mean displacement of T cells as a function of √(time). The slope of this graph was used to determine the motility coefficient, a measure of the ability of a cell to move away from its starting position (Table 1).6,30 (C) Turning angles of individual T cells measured as the change in direction of movement occurring between successive frames. (D) Top, a cumulative distribution plot of the meandering index (displacement/path length) of T cells of the indicated genotypes. Bottom, the median meandering index (± interquartile range). The meandering index is a measure of the straightness of track. WT v Rac1T: 13 videos analyzed from 6 independent experiments; WT v Rac2−/−: 14 videos analyzed from 9 independent experiments; WT v DKO: 7 videos analyzed from 4 independent experiments. *P < .001.
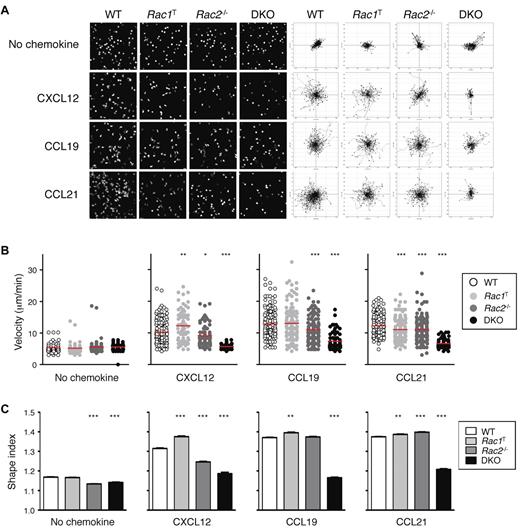
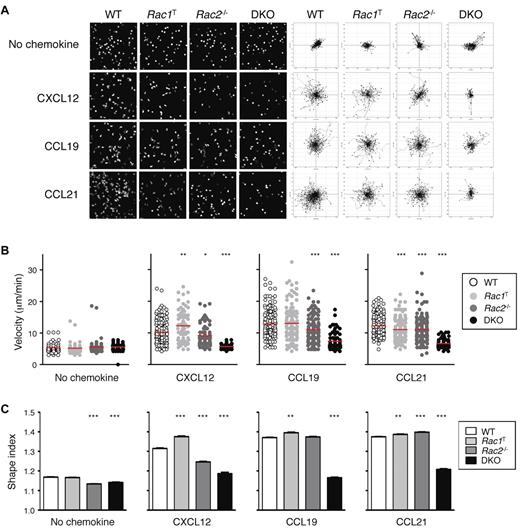
Because interstitial motility is in part dependent on chemokines, we analyzed the chemokine-induced movement (chemokinesis) of Rac-deficient T cells. We found that in the absence of Rac2, the motility of T cells in response to CXCL12, CCL19, and CCL21 was partially reduced, whereas DKO T cells were almost completely unresponsive (Figure 5A-B; supplemental Videos 8-9). Rac1T T cells showed only minor changes in motility. The reduced chemokinesis of DKO T cells was also associated with an inability of the cells to adopt an elongated polarized shape (Figure 5C). In conclusion, Rac1 and Rac2 are essential for chemokine-induced movement, which likely accounts for the reduction in interstitial motility in LNs of Rac-deficient T cells.
Defective chemokinesis in Rac-deficient T cells. (A) CD4+ T cells from mice of the indicated genotypes were cultured in the presence of CXCL12, CCL19, or CCL21 or with no chemokine, and their EYFP fluorescence was recorded by video microscopy. Images on the left show a snapshot from the videos. Flower plots on the right show migration tracks of individual cells, with the starting point of each track placed at the origin (0, 0). (B) Scatter plots of mean velocities of individual cells of the indicated genotypes taken from the videos described in panel A. Red bars indicate the average of these velocities. (C) Mean ± SEM shape index of cells of the indicated genotypes responding to the indicted stimulus derived from the videos described in panel A. A circle has a shape index of 1.0; any distortion from a perfect circle results in a shape index of > 1.0. Data are from 1 representative experiment of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Defective chemokinesis in Rac-deficient T cells. (A) CD4+ T cells from mice of the indicated genotypes were cultured in the presence of CXCL12, CCL19, or CCL21 or with no chemokine, and their EYFP fluorescence was recorded by video microscopy. Images on the left show a snapshot from the videos. Flower plots on the right show migration tracks of individual cells, with the starting point of each track placed at the origin (0, 0). (B) Scatter plots of mean velocities of individual cells of the indicated genotypes taken from the videos described in panel A. Red bars indicate the average of these velocities. (C) Mean ± SEM shape index of cells of the indicated genotypes responding to the indicted stimulus derived from the videos described in panel A. A circle has a shape index of 1.0; any distortion from a perfect circle results in a shape index of > 1.0. Data are from 1 representative experiment of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Parenchymal motility in LNs is mediated by at least 2 receptor classes, CCR7 and other, currently unknown, Gαi-protein-coupled receptor(s).3,4,6 To examine the roles of Rac1 and Rac2 in signaling from these latter receptors, we transferred T cells into plt/plt mice, which lack the CCR7 ligands CCL21 and CCL19 in LNs. By using 2-photon IVM to image interstitial movement, we found that WT T cells, as well as T cells deficient in either Rac1 or Rac2, moved at similar velocities of approximately 10 μm/min (supplemental Figure 4; Table 1). Taken together with studies of DKO T cells, these results show that in the less promigratory environment of plt/plt mice, T cells require only one Rac isoform for efficient motility, and thus that these GTPases are functionally redundant in transducing the unidentified migratory signals in plt/plt LNs. However, in WT LNs, where CCL19 and CCL21 also contribute to interstitial motility, both GTPases are required for normal movement because loss of only Rac2 partially reduces mean velocities.
Rac1 and Rac2 are required for efficient egress from LNs
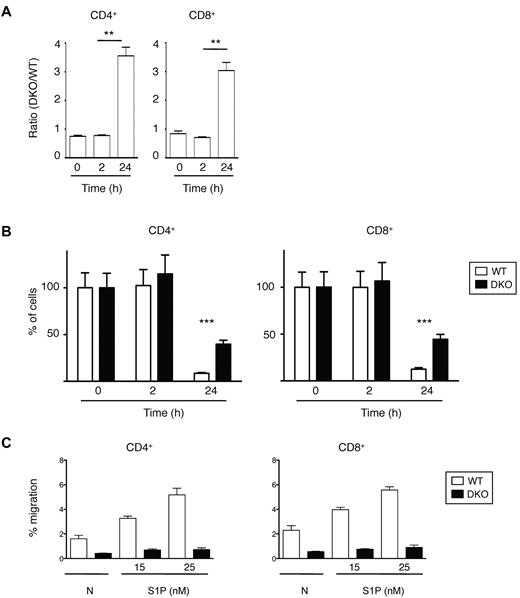
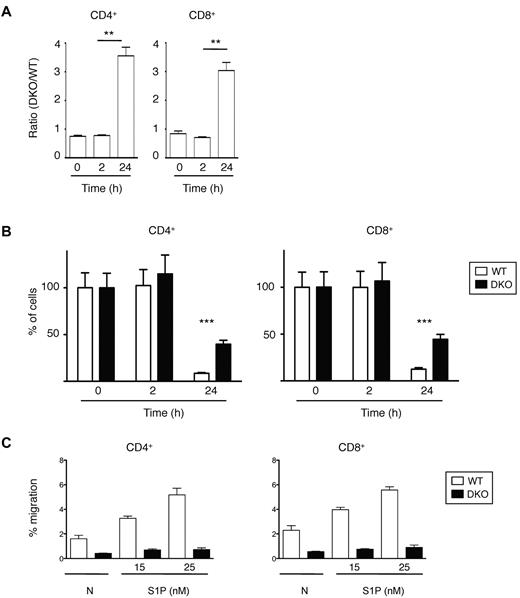
Finally, we investigated the role of Rac1 and Rac2 in the last step of lymphocyte recirculation, egress from LNs. We transferred a mixture of WT and DKO T cells into C57BL/6 mice and 12 hours later injected anti-CD62L antibody into the mice to prevent further entry of lymphocytes into LNs and measured the number of remaining T cells 0, 2, and 24 hours after antibody injection. This study showed that both CD4+ and CD8+ DKO T cells egressed from the LNs less efficiently than WT cells (Figure 6A-B). This defect may be the result of a role for Rac1 and Rac2 in transducing signals from S1P1, because both CD4+ and CD8+ DKO T cells were defective in S1P-induced chemotaxis (Figure 6C).
Lymphocyte egress from LNs. (A) A mixture of WT and DKO T cells were transferred into C57BL/6 mice, and 12 hours later Mel-14 was injected into the mice to stop further entry into LNs. Graph shows mean ± SEM ratio of DKO to WT CD4+ or CD8+ T cells in LNs as a function of time after Mel-14 injection. The ratio was normalized to the ratio of DKO to WT T cells in the cells injected into the mice. (B) Graph shows percentage (mean ± SEM) CD4+ or CD8+ T cells remaining as a function of time after Mel-14 injection, normalized to the number of cells at 0 hours, which was set to 100%. Data in panels A and B are from 2 independent experiments. (C) Graph shows mean ± SEM percentage migration of WT or DKO EYFP+ T cells (CD4+ or CD8+) in a Transwell assay in response to S1P at the indicated concentrations, or in the absence of chemokine (N). Data are from 1 representative experiment of 2 independent experiments. *P < .01; ***P < .001.
Lymphocyte egress from LNs. (A) A mixture of WT and DKO T cells were transferred into C57BL/6 mice, and 12 hours later Mel-14 was injected into the mice to stop further entry into LNs. Graph shows mean ± SEM ratio of DKO to WT CD4+ or CD8+ T cells in LNs as a function of time after Mel-14 injection. The ratio was normalized to the ratio of DKO to WT T cells in the cells injected into the mice. (B) Graph shows percentage (mean ± SEM) CD4+ or CD8+ T cells remaining as a function of time after Mel-14 injection, normalized to the number of cells at 0 hours, which was set to 100%. Data in panels A and B are from 2 independent experiments. (C) Graph shows mean ± SEM percentage migration of WT or DKO EYFP+ T cells (CD4+ or CD8+) in a Transwell assay in response to S1P at the indicated concentrations, or in the absence of chemokine (N). Data are from 1 representative experiment of 2 independent experiments. *P < .01; ***P < .001.
Rac1 and Rac2 transduce chemokine receptor signals leading to rearrangement of the cytoskeleton
Rac GTPases have been postulated to contribute to migration and adhesion through controlling rearrangement of the actin cytoskeleton.10,11 To investigate this in primary T cells, we measured levels of polymerized F-actin in Rac-deficient T cells after chemokine stimulation. Analysis showed that unstimulated DKO T cells had reduced levels of F-actin compared with WT T cells (Figure 7A). After stimulation with CCL19, CCL21, or CXCL12, F-actin levels increased in WT T cells but were partially reduced in Rac1T and Rac2−/− cells and greatly reduced in DKO T cells. Thus, Rac1 and Rac2 are required to transduce chemokine receptor signals leading to actin polymerization, and once again show functional redundancy in this process.
Rac GTPases required for chemokine-induced cytoskeletal reorganization. (A) Mean ± SEM F-actin in CD4+ T cells of the indicated genotypes stimulated with CXCL12, CCL19, or CCL21 (200 ng/mL) or not stimulated. All F-actin measurements were normalized to F-actin content of unstimulated WT T cells. Data are from 4 independent experiments. (B) Mean ± SEM pERM in CD4+ T cells of the indicated genotypes stimulated with CCL21 (500 ng/mL) for the indicated times. All pERM measurements were normalized to WT T cells at time = 0 seconds. Data are from 3 independent experiments. *P < .05; **P < .01; ***P < .001.
Rac GTPases required for chemokine-induced cytoskeletal reorganization. (A) Mean ± SEM F-actin in CD4+ T cells of the indicated genotypes stimulated with CXCL12, CCL19, or CCL21 (200 ng/mL) or not stimulated. All F-actin measurements were normalized to F-actin content of unstimulated WT T cells. Data are from 4 independent experiments. (B) Mean ± SEM pERM in CD4+ T cells of the indicated genotypes stimulated with CCL21 (500 ng/mL) for the indicated times. All pERM measurements were normalized to WT T cells at time = 0 seconds. Data are from 3 independent experiments. *P < .05; **P < .01; ***P < .001.
Finally, we examined the role of Rac GTPases in the phosphorylation of ERM (ie, ezrin/radixin/moesin) proteins that, when phosphorylated, are able to bind both cortical actin and the cytoplasmic domain of cell surface proteins such as CD43, resulting in cellular rigidity. Faure et al31 showed that Rac GTPases transduce TCR signals, leading to transient dephosphorylation of ERM proteins, thereby causing detachment of the cortical actin from the plasma membrane and hence increased cell deformability. Analysis of the same process in response to CCL21 also showed a transient ERM dephosphorylation that is reduced in DKO T cells (Figure 7B). Thus, Rac GTPases may contribute to chemokine-induced cell deformability.
Discussion
In this study we report the first analysis of primary T cells deficient in both Rac1 and Rac2. Because mouse T cells express little or no detectable Rac3, the T cells we have generated in these mice are completely devoid of Rac GTPases. We were surprised to discover that although loss of Rac GTPases caused decreased T-cell numbers, a large proportion of the cells were able to survive. Previous studies have shown that deletion of Rac1 and Rac2 in the T-cell lineage leads to a very strong developmental arrest in the thymus, which is consistent with critical roles of the GTPases in transducing signals from the pre-TCR and TCR.13,29 Because TCR signaling is essential for survival of naive T cells,32 we expected that Rac-deficient T cells would be unable to survive. The observation that such cells can survive suggests that the signaling function of Rac GTPases downstream of the TCR may be different in the context of TCR signals leading to T-cell survival compared with TCR signals driving positive selection in thymocytes.
In contrast to T-cell survival, efficient recirculation through SLOs required Rac1 and Rac2. By using adoptive transfer experiments we were able to show that Rac-deficient T cells were defective in their ability to home to LNs and to the white pulp of the spleen, with clear defects in the entry of T cells into LNs, movement within them and egress from them. Analysis of T-cell entry into LNs showed that mutant T cells accumulated at the HEV, which further study showed is likely attributable to a combination of defects in integrin and chemokine receptor function.
Rac-deficient T cells were defective in chemokine-induced adhesion to ICAM-1 and in transendothelial migration, an LFA-1–dependent process,33 and adhered less firmly to endothelium. Because mutant T cells were able to attach to endothelium under low shear stress, these results indicate that the initial activation of integrins is occurring normally in Rac-deficient T cells but that subsequent adhesion strengthening is defective. Studies in human T cells have suggested that Rac1 is required for the rapid chemokine-induced activation of LFA-1 into its high affinity state,16 which appears to be in contrast to the results shown here, where initial chemokine-induced attachment of Rac-deficient T cells to endothelial cells occurred normally. This apparent discrepancy may be the result of differences between human and mouse T cells or the use of a dominant-negative Rac1 protein, which could inhibit GEFs that act on GTPases other than just Rac proteins.
It remains unclear which GEFs for Rac GTPases are required to transduce chemokine receptor signals via Rac1 and Rac2 to integrin activation. In contrast to Rac-deficient cells, mouse T cells deficient in DOCK2 or Tiam1 show normal chemokine-induced adhesion to ICAM-1 and transendothelial migration.19,20,22 Reports that Vav1 may transduce signals from LFA-1 and that expression of a dominant-negative Vav1 in human T-cell lines inhibits CXCL12-mediated adhesion through VLA-4 suggest that Vav-family GEFs may be important in this process.14,34
Our studies have shown a critical requirement for Rac GTPases in chemokine-induced motility. By using in vitro assays we showed that DKO T cells were severely compromised in their ability to undergo either chemotaxis or chemokinesis in response to CCR7 or CXCR4 stimulation. Furthermore, Rac-deficient T cells showed greatly decreased lateral mobility both on top of and underneath an endothelial monolayer in flow chamber assays, processes dependent on chemokine receptor signaling. The use of IVM confirmed that DKO T cells did not move away rapidly from HEV even after successful transmigration. Finally, we showed that interstitial mobility of T cells within the parenchyma of LNs, a process partly dependent on CCR7 signaling, was strongly inhibited in the absence of Rac1 and Rac2. The importance of Rac GTPases for cell motility has been demonstrated in other cell types. Rac2−/− B cells show decreased chemotaxis in response to CXCL12 and CXCL13,35 and neutrophils deficient in Rac1 and Rac2 or both, are defective to chemotaxis in response to fMLP, interluekin-8 and leukotriene B4.25,36,37 Nonetheless, despite the importance of Rac GTPases for migration in some cell types, this is not universally true. Macrophages deficient in either Rac1, Rac2, or both are able to migrate at normal speeds in response to colony-stimulating factor–1, albeit with abnormal morphology.38,39 Thus, it is not possible to generalize conclusions from one cell type to another, which emphasizes the importance of determining the contribution of these GTPases to migration in each cell type individually.
Chemokine receptor signals leading to Rac1 and Rac2 activation and hence T-cell motility may be transduced, at least in part, via DOCK2, because of the similarities in phenotype between the respective genetic deficiencies. Like Rac-deficient T cells, DOCK2−/− T cells are defective in chemokine-induced chemotaxis in vitro, in migration both on top of and underneath an endothelial cell monolayer, and in interstitial migration.18-21 Furthermore, DOCK2−/− lymphocytes showed reduced CXCL12-induced Rac1 activation.18 However, T cells deficient in Tiam1, another Rac-specific GEF, also show reduced chemokine-induced chemotaxis, motility on endothelial cells and homing to SLOs, suggesting that chemokine receptors may use more than one GEF to activate Rac proteins.22 Finally, it should be noted that despite the similarities in phenotype, the chemotactic defects in DOCK2−/− and Tiam1−/− T cells may not be the result of GEF functions of these proteins because these are both large proteins (212 kDa and 178 kDa, respectively) and thus may have non-GEF activities, as has been observed for the Vav1 GEF.23 A resolution of this issue will require analysis of T cells expressing GEF-inactive forms of DOCK2 and Tiam1.
DKO T cells showed a reduction in interstitial motility within LNs, greater than the reduction seen in plt/plt mice, implying that Rac1 and Rac2 transduce motility signals not only from CCR7, but also from the unknown receptors that drive residual motility in plt/plt mice.3,4,6 Like CCR7 these latter receptors also signal via Gαi.3 Similar to DOCK2−/− T cells,21 DKO T cells also were defective in LN egress and S1P-induced chemotaxis, suggesting that S1P1, another Gαi-coupled receptor, also transduces chemotactic signals via DOCK2 and Rac1/Rac2. Taken together, these results identify common DOCK2- and Rac-dependent signaling mechanisms in T cells from several Gαi-coupled receptors.
Our analysis demonstrates strong functional redundancy between Rac1 and Rac2, with much stronger phenotypes seen in DKO T cells compared with the single mutants in chemotaxis, chemokinesis, interstitial motility, adhesion, and actin polymerization. T cells missing either GTPase alone typically had mild phenotypes, with Rac2 deficiency always showing the stronger effect. Taken together we conclude that in the processes examined here, Rac1 and Rac2 have highly redundant functions, with Rac2 usually playing a more prominent role, perhaps because T cells express more Rac2 than Rac1.40
In summary, our studies show that Rac1 and Rac2 play critical roles in T-cell recirculation, affecting at least 5 distinct steps: firm adhesion to endothelium, lateral migration on endothelium, transmigration, interstitial migration within the LN, and egress out of lymphoid tissue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nigel Killeen, Dimitris Kioussis, and David Williams for mice; Biological Services at the National Institute for Medical Research for animal husbandry; and Sarah Henrickson and Ulrich H. von Andrian for the CellTracker tool.
M.F. was a recipient of an Association pour la Recherche sur le Cancer (ARC) fellowship, a European Molecular Biology Organization long-term fellowship, and a EU Marie Curie fellowship. A.Z., C.D., and V.T. were funded by the Medical Research Council program number U117527252; and M.H. and J.V.S. were supported by the Swiss National Foundation and a European Union Marie Curie Excellence grant.
Authorship
Contribution: M.F. and M.H. designed and performed research, analyzed and interpreted data, and wrote the paper; A.Z. and C.D. performed research and analyzed data; R.L. designed research; and J.V.S. and V.L.J.T. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Victor L. J. Tybulewicz, MRC National Institute for Medical Research, The Ridgeway, Mill Hill, London, NW7 1AA, United Kingdom; e-mail: vtybule@nimr.mrc.ac.uk.