Abstract
Differentiation of human pluripotent stem cells (hPSCs) into functional cell types is a crucial step in cell therapy. In the present study, we demonstrate that functional CD34+ progenitor cells can be efficiently produced from human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) by combined modulation of 2 signaling pathways. A higher proportion of CD34+ cells (∼ 20%) could be derived from hPSCs by inhibition of mitogen-activated protein kinase (MAPK) extracellular signal-regulated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling and activation of bone morphogenic protein-4 (BMP4) signaling. hPSC-derived CD34+ progenitor cells further developed to endothelial and smooth muscle cells with functionality. Moreover, they contributed directly to neovasculogenesis in ischemic mouse hind limbs, thereby resulting in improved blood perfusion and limb salvage. Our results suggest that combined modulation of signaling pathways may be an efficient means of differentiating hPSCs into functional CD34+ progenitor cells.
Introduction
Human embryonic stem cells (hESCs) derived from an early embryo can self-renew indefinitely and differentiate into a variety of cell types.1 It has been reported that the “stemness” of hESCs is likely maintained through the harmonious actions of signaling pathway networks.2 Basic fibroblast growth factor (bFGF) is essential for maintaining the stemness of hESCs by highly activating mitogen-activated protein kinase (MAPK) extracellular signal-regulated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling, which plays an important role in the stemness of hESCs.3 Stemness of hESCs is also supported by bFGF-mediated regulation of transforming growth factor-β (TGF-β) signaling4 ; activation of the TGF-β/activin/nodal signaling pathway is required to maintain stemness in cooperation with the FGF signaling pathway, whereas its inhibition results in differentiation of hESCs.5,6 The effect of Wnt signaling on stemness of hESCs is still controversial. Activation of the Wnt pathway by 6-bromoindirubin-3-oxmie, a specific inhibitor of glycogen synthase-3, sustains the undifferentiated status of hESCs.7 However, activation of Wnt signaling is insufficient to maintain the undifferentiated status of hESCs, because a canonical Wnt signaling is highly activated during differentiation.8
The stemness of human induced pluripotent stem cells (hiPSCs), like that of hESCs, seems to be maintained by coordinated networks of signaling molecules, although few differences are observed in the gene expression profile.9,10 Thus, the stemness and differentiation of hESCs and hiPSCs is regulated by complex networks of signaling pathways. It is likely that the modulation of these signaling pathways can induce differentiation of hESCs into a specialized cell type. In fact, bone morphogenic protein 2/4 (BMP2/4), a member of the TGFβ superfamily, could differentiate hESCs into trophoblasts, primitive endoderm cells, and mesodermal cells.11-13 However, it has been reported that BMP4 is required for sustaining stemness of mouse ESCs by blocking neural differentiation.14 hESCs could be differentiated to definitive endoderm cells by activation of activin/nodal signaling and suppression of phosphoinositide 3-kinase (PI3K) signaling.15 Dual inhibition of SMAD signaling by treatment with Noggin and SB431542 resulted in differentiation of hESCs and hiPSCs into neural cells.16 Many studies have tried to isolate specialized cell types from spontaneously differentiated cells via formation of hESC-derived embryoid bodies using antibodies against cell-type-specific surface markers.17,18 However, spontaneous differentiation remains inefficient, in that hPSCs cannot be guided toward a specialized lineage at the initial commitment step.
The hPSCs provide a possibility that degenerative or damaged tissues can be replaced with hPSC-derived functional cells. A paucity of number and activity of endothelial progenitor cells is correlated with cardiovascular diseases such hypercholesterolemia, hypertension, and diabetes mellitus.19 Therefore, endothelial progenitor cells are able to be used for curing such cardiovascular diseases. In fact, endothelial progenitor cells derived from bone marrow or cord blood were effective on vasculogenesis in ischemic diseases.20,21 Therefore, it is suggested that transplantation of vascular cells into ischemic regions may enhance restoration of tissue revascularization.22 Recently, injection of hESC-derived endothelial cells was found to salvage ischemic hind limbs and enhance blood perfusions.23,24 However, there are still limitations of cell therapy using hESC-derived cells, including low efficiency of differentiation into specialized progenitors and the use of animal sources such as animal serum and feeder cells. In an effort to address these issues, we developed a simple and efficient method by which hESCs and hiPSCs are directly differentiated to functional CD34+ progenitor cells through regulation of signaling pathways. Both hESC- and hiPSC-derived CD34+ cells were differentiated into functional endothelial cells, and contributed to blood perfusion and limb salvage through the neovasculogenesis in hind limb ischemic mice, respectively.
Methods
Maintenance and feeder-free culture of hESCs and hiPSCs
The hESCs (CHA4-hES,25 supplemental Figure 1) and hiPSCs (supplemental Figure 2) were cultured in ESC medium on mitomycin C (Sigma-Aldrich)–treated STO (ATCC no. CRL-1503) feeders at 37°C, in air containing 5% CO2. The ESC medium consisted of Dulbecco modified Eagle medium (DMEM)/F12 medium containing 20% knockout serum replacement, 1% nonessential amino acids, 0.1mM β-mercaptoethanol, and 4 ng/mL of bFGF (all from Invitrogen). For feeder-free culture, hPSCs were maintained on Matrigel (BD Biosciences)–coated culture dishes in STO-conditioned medium.
RT-PCR and real-time RT-PCR
For extracting total RNA, hPSCs or mouse tissues were homogenized in TRIzol (Invitrogen) according to the manufacturer's protocol. Then, 1 μg of total RNA was used to generate first-strand cDNA using Superscript II reverse transcriptase (RT) (Invitrogen), and cDNA was amplified by polymerase chain reaction (PCR) using the PCR PreMix (Genet Bio, Daejeon, Korea). The specific primers used in this study are listed in supplemental Tables 1 to 3. The RT-PCR reaction was performed with an initial step at 95°C for 5 minutes, followed by 25 to 35 cycles of 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C, and a final elongation step at 72°C for 5 minutes. The PCR products were resolved on a 2% agarose gel by electrophoresis. Relative expression levels among respective genes were analyzed by using an iCycler iQ5 real-time detection system (Bio-Rad Laboratories). All reactions were performed in triplicate. For comparative quantification, the expression level of respective genes was normalized to that of GAPDH, and expressed as a fold change relative to the expression level in undifferentiated hESCs. The sample ΔCt (SΔCt) value was calculated from the difference between the Ct values of GAPDH and the target genes. The relative gene expression levels between the sample and control were determined using the formula 2−(SΔCt−CΔCt).
Immunostaining
The cells were washed with phosphate-buffered saline (PBS) and fixed in 4% formaldehyde at room temperature for 15 minutes. For detection of nuclear proteins, the hPSCs were permeabilized with 0.1% Triton X-100 in PBS, and blocked with 4% normal goat serum or fetal bovine serum (FBS) for 1 hour at room temperature. After that, antibodies against octamer-binding transcription factor 4 (OCT4; R&D Systems, 1:300), T (R&D Systems, 1:100), GATA2 (R&D Systems, 1:100), SSEA-4 (R&D Systems, 1:300), PECAM-1 (R&D Systems, 1:100), Von Willebrand factor (VWF) (Abcam, 1:100), KDR (Cell Signaling Technologies, 1:100), VE-cadherin (R&D Systems, 1:100), α-smooth muscle actin (α-SMA; R&D Systems, 1:100), and calponin (Abcam, 1:100) were diluted with blocking solution and incubated with the prepared cells at 4°C overnight. Finally, the cells were washed several times with PBST (0.1% Tween-20 in PBS) and incubated with Alexa Fluor 488– or 594–conjugated secondary antibodies (Invitrogen). Immunostained cells were observed on a fluorescence microscope (Olympus) or a Zeiss LSM 510 confocal microscope (Carl Zeiss).
Immunohistochemistry
For histologic analysis, mice were anesthetized by intramuscular injection (80 mg/kg ketamine and 12 mg/kg xylazine), and then fixed by vascular perfusion of 1% paraformaldehyde in PBS. Tissues were harvested and embedded in cryofreezing medium. Cryosections (12 μm thickness) were incubated in PBST (0.3% Triton X-100 in PBS) containing 5% donkey serum (Jackson ImmunoResearch Laboratories) at room temperature for 1 hour. After blocking, the samples were incubated at 4°C overnight with a mouse anti–human CD31 antibody (Abcam, 1:200) combined with a hamster anti–mouse CD31 antibody (Chemicon International, 1:200). After several washes in PBST, the samples were incubated for 1 hour at room temperature with fluorescent-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, 1:500). Nuclei were stained with 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI, 1 μg/mL in PBS; Invitrogen) at room temperature for 30 minutes. Signals were visualized on a confocal microscope equipped with argon and helium-neon lasers (model LSM 510, Zeiss). For determining functionality of blood vessels, fluorescein Bauhinia purpurea lectin (2 mg/mL; FL-1281, Vector Laboratories) was intravenously injected into ischemic mice at a volume of 3.75 μL/g body weight 7 days after injection of hPSC-derived CD34+ cells. Ten minutes after injection of lectin, the tissue samples were prepared and immunostained with anti–human CD31 antibody and anti–mouse CD31 antibody, as described above.
Isolation of CD34+ cells by magnetic sorting
To dissociate differentiated hPSCs into single cells, differentiated hPSCs were treated with TrypLE Express (Invitrogen) for 10 minutes at 37°C and, after gentle pipetting, passed through 40-μm cell strainers (BD Biosciences). CD34+ cells were then isolated by MACS MagneticBead columns (Miltenyi Biotec) using an antibody against CD34, according to the manufacturer's instructions.
Acetylated low-density lipoprotein uptake assay and vascular tube-like structure formation assay
For the low-density lipoprotein (LDL) uptake assay, endothelial cells derived from CD34+ cells were incubated with 10 μg/mL of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (Dil)–labeled acetylated LDL (Invitrogen) for 5 hours. Red fluorescent signals were detected with a fluorescence microscope. To assess the formation of vascular tube-like structures, 1 × 105 endothelial cells were incubated on Matrigel matrix (BD Biosciences) in endothelial cell growth medium-2 (EGM-2) supplemented with vascular endothelial growth factor-1 (VEGF-A) for 24 hours. The vascular tube-like structures were observed with an inverted microscope.
Colony-forming unit assay
A colony-forming unit (CFU) assay was used to determine whether CD34+ cells could differentiate to hematopoietic cells. Approximately 1 × 105 CD34+ cells were placed on a 35-mm culture dish containing MethoCult GF H4434 (StemCell Technologies), which is composed of 1% methylcellulose, 30% FBS, 1% BSA, 0.1mM 2-mercaptoethanol, 2mM l-glutamine, 50 ng/mL of recombinant human stem cell factor, 10 ng/mL of recombinant human granulocyte monocyte–colony-stimulating growth factor, 10 ng/mL of recombinant human interleukin-3, and 3 U/mL of recombinant human erythropoietin. The various hematopoietic colonies were enumerated and identified at days 14 to 21.
Flow cytometry
Cells were labeled with antibodies against CD105-APC, CD31-PE, CD45-FITC, CD73-PE, CD90-APC, and CD34-APC (all from BD Pharmingen) at 4°C for 30 minutes. After being washed twice with PBS containing 1% FBS, the antibody-labeled cells were analyzed with a flow cytometer (LSRII, Becton Dickinson), according to the manufacturer's instructions. The data were analyzed using FlowJo Version 7.2.5 software (TreeStar).
In vivo Matrigel assay
A total of 1 × 106 hESC-derived CD34+ cells were mixed with Matrigel (100 μL, BD Biosciences) and then subcutaneously injected into the flank region of an 8-week-old BALB/cByJ athymic nude (CbyJ.Cg-Foxn1nu/J) mouse. Immunohistochemistry was performed 10 days after implantation.
Preparation of ischemic hind-limb mice
BalB/cAnNCriBgi-nu nude male mice were purchased from Charles River Japan (Yokohama, Japan). Seven- to 8-week-old mice (15-20 g body weight) were used to make the ischemia model. Hind-limb ischemia was induced by ligation and excision of the right femoral artery and vein under ketamine-xylazine anesthesia. Animal care and experimental procedures were performed under the approval of the animal care committees of KAIST. For therapeutic angiogenesis studies, mice were divided into 3 groups after induction of ischemia for intramuscular injection with culture medium (20 μL), CD34+ culture (1 × 106cells/20 μL medium) or CD34− culture (1 × 106cells/20 μL medium). Serial near-infrared fluorescence imaging was performed immediately after surgery and then on postoperative days (POD) 3 and 7.
Analysis of near-infrared fluorescence imaging
To measure tissue perfusion, we performed indocyanine green perfusion imaging using a near-infrared fluorescence imaging system (Vieworks), as described previously.26 Detailed experimental procedures are described in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Statistical analysis
The statistical significance of the real-time RT-PCR data rate was evaluated by using one-way ANOVA and Bonferroni post-hoc tests, where values of P < .05 were considered significant. For the analysis of the perfusion rates and necrosis probability in the ischemic hind limbs, ANOVA and Scheffe post-hoc test were performed.
Results
Synergistic differentiation of hPSCs into mesoderm-lineage cells by regulation of MEK/ERK and BMP4 signaling
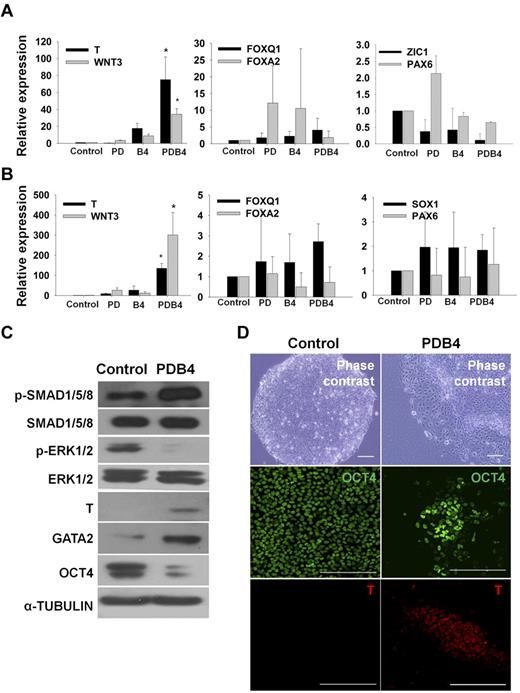
In this study, we have established a novel procedure for direct differentiation of hESCs to mesoderm-lineage cells by combined regulation of the MEK/ERK and BMP4 signaling pathways (supplemental Figure 3). When hESCs were treated with PD98059 and BMP4 (PDB4) for 3 days, expression of mesoderm marker genes (T and WNT3) were significantly increased compared with that of PD98059 or BMP4 treatment alone (Figure 1A). However, definitive endodermal (FOXQ1 and FOXA2) and ectodermal (ZIC1, SOX1, and PAX6) genes were not significantly changed (Figure 1A). Similarly, hiPSCs could be also differentiated into mesoderm lineage cells by treatment with PD98059 and BMP4 (Figure 1B). The expression of the mesoderm markers was confirmed at the protein level by western-blot analysis (Figure 1C). As expected, PDB4 treatment enhanced phosphorylation of SMAD1/5/8 and reduced phosphorylation of ERK1/2 in hESCs. Moreover, the mesoderm markers T and GATA2 were up-regulated, whereas the expression of the stem-cell marker OCT4 was down-regulated in the PDB4-treated sample (Figure 1C). In addition, the expression of T was observed in PDB4-treated hESCs (Figure 1D) and hiPSCs (supplemental Figure 4). These results demonstrate that the mesoderm-lineage differentiation of hESCs and hiPSs could be committed by coordinated regulation of the MEK/ERK and BMP4 signaling pathways.
Enhanced expression of mesoderm marker genes in hESCs and hiPSCs by treating PD98059 and BMP4. (A) and (B) Relative expression levels of mesoderm (T and WNT3), endoderm (FOXA2 and FOXQ1), and ectoderm(ZIC1, SOX1, and PAX6) marker genes between experimental groups in hESCs and hiPSCs. The values are the mean ± SD of 3 independent experiments. A P value < .05 was considered to be statistically significant (*P < .05; n = 3). (C) Western blot analysis for phosphorylation of ERK1/2 and SMAD1/5/8 and expression of a stem cell marker (OCT4) and mesoderm markers (T, GATA2) in undifferentiated hESCs (control) and PDB4-treated hESCs. (D) Immunostaining for a stem cell marker (OCT4) and a mesoderm marker (T) in undifferentiated hESCs (control) and PDB4-treated hESCs. Scale bar is 200 μm. Abbreviations: Control, untreated hESCs; PD, PD98059; B4, BMP4; PDB4, hESCs treated with PD98059 and BMP4.
Enhanced expression of mesoderm marker genes in hESCs and hiPSCs by treating PD98059 and BMP4. (A) and (B) Relative expression levels of mesoderm (T and WNT3), endoderm (FOXA2 and FOXQ1), and ectoderm(ZIC1, SOX1, and PAX6) marker genes between experimental groups in hESCs and hiPSCs. The values are the mean ± SD of 3 independent experiments. A P value < .05 was considered to be statistically significant (*P < .05; n = 3). (C) Western blot analysis for phosphorylation of ERK1/2 and SMAD1/5/8 and expression of a stem cell marker (OCT4) and mesoderm markers (T, GATA2) in undifferentiated hESCs (control) and PDB4-treated hESCs. (D) Immunostaining for a stem cell marker (OCT4) and a mesoderm marker (T) in undifferentiated hESCs (control) and PDB4-treated hESCs. Scale bar is 200 μm. Abbreviations: Control, untreated hESCs; PD, PD98059; B4, BMP4; PDB4, hESCs treated with PD98059 and BMP4.
Differentiation of PDB4-treated hESCs and hiPSCs into CD34+ progenitor cells
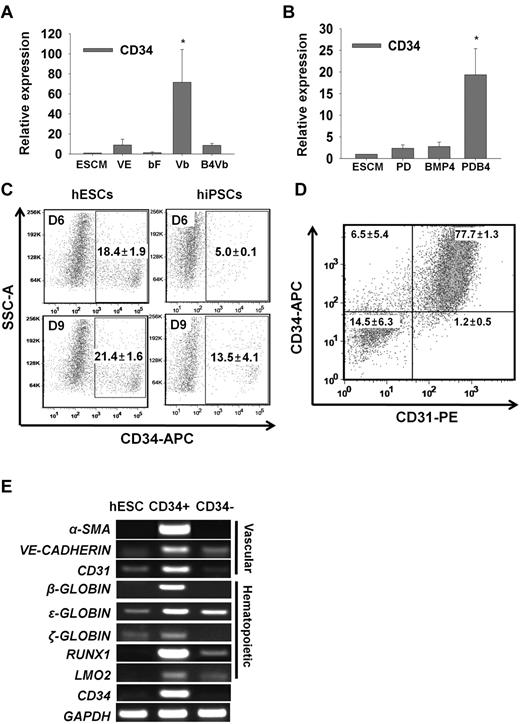
To further develop the PDB4-treated hESCs into the CD34+ cells downstream of mesodermal lineage, we first investigated the effects of growth factors. When PDB4-treated hESCs were cultured in ESC medium supplemented with VEGF-A and bFGF (Vb) for 6 days, the expression of a vascular/hematopoietic progenitor marker gene CD34 was remarkably enhanced compared with VEGF-A (VE) or bFGF (bF) treatment alone (Figure 2A). It has been determined that BMP4 is effective on the induction of hESCs into hematopoietic and endothelial cells.18,27,28 However, in this study, additional BMP4 did not facilitate differentiation of the mesodermal cells into CD34+ cells (Figure 2A), although BMP4 was essential for mesoderm induction in hESCs, as shown in Figure 1. Thus, bFGF and VEGF-A were effective in differentiating the PDB4-treated cells into CD34+ cells. To determine whether the expression level of CD34 could be influenced by upstream protocols for mesodermal induction, PD-, BMP4-, and PDB4-treated hESCs were cultured in ESC medium supplemented with Vb for 6 days (Figure 2B). The PDB4 group showed a higher transcriptional expression of CD34 than the other groups, demonstrating that the expression of CD34 may be proportional to the strength of mesodermal induction. Other vascular/hematopoietic progenitor marker genes such as RUNX1, KDR (VEGFR2), and VE-CADHERIN were also highly transcribed in Vb-treated samples (supplemental Figure 5). There was a minor difference in the proportion of CD34+ cells generated from hESCs and hiPSCs using our protocols (Figure 2C). The proportion of CD34+ cells was approximately 20% at 9 days of vascular induction in hESC group. A lower proportion (∼13%) of hiPSC-derived mesodermal cells reached CD34+ cells at 9 days of vascular induction. Next, CD34+ cells were isolated by a magnetic cell sorter (MACS) using an antibody against CD34. Most of the CD34+ cells coexpressed CD31, and the proportions of CD34+CD31− and CD34−CD31+ cells were approximately 6.5% and 1.2%, respectively (Figure 2D). As shown in Figure 2E, the CD34+ cells also expressed other vascular marker genes (α-SMA, VE-CADHERIN, and CD31) and hematopoietic marker genes (β-, ϵ-, and ζ-GLOBIN, RUNX1, and LMO2) at the transcriptional level. The results suggest that hESC-derived CD34+ cells can differentiate into hematopoietic- and vascular-lineage cells.
Generation of CD34+ cells from PDB4-treated hESCs after treatment with VEGF-A and bFGF. (A) and (B) Relative mRNA levels of CD34 between experimental groups. Error bars indicate the mean ± SD of 3 independent experiments. A P value < .05 was considered to be statistically significant (*P < .05; n = 3). (C) Proportion of CD34+ cells derived from CHA-hES4 and hiPSCs. PDB4-treated hESCs were cultured in VEGF-A and bFGF-containing medium for 6 and 9 days. The percentage of CD34+ cells (n = 3) is indicated as mean ± SD. (D) Coexpression of CD34 and CD31 in isolated CD34+ cells (n = 2). (E) Transcriptional expression of vascular-lineage genes (α-SMA, VE-CADHERIN, and CD31) and hematopoietic-lineage genes (β-GLOBIN, ϵ-GLOBIN, ζ-GLOBIN, RUNX1, and LMO2) in CD34+ cells. Abbreviations: Control, untreated hESCs; VE, VEGF-A; bFGF, bFGF; Vb, VEGF-A and bFGF; B4Vb, BMP4, VEGF-A and bFGF.
Generation of CD34+ cells from PDB4-treated hESCs after treatment with VEGF-A and bFGF. (A) and (B) Relative mRNA levels of CD34 between experimental groups. Error bars indicate the mean ± SD of 3 independent experiments. A P value < .05 was considered to be statistically significant (*P < .05; n = 3). (C) Proportion of CD34+ cells derived from CHA-hES4 and hiPSCs. PDB4-treated hESCs were cultured in VEGF-A and bFGF-containing medium for 6 and 9 days. The percentage of CD34+ cells (n = 3) is indicated as mean ± SD. (D) Coexpression of CD34 and CD31 in isolated CD34+ cells (n = 2). (E) Transcriptional expression of vascular-lineage genes (α-SMA, VE-CADHERIN, and CD31) and hematopoietic-lineage genes (β-GLOBIN, ϵ-GLOBIN, ζ-GLOBIN, RUNX1, and LMO2) in CD34+ cells. Abbreviations: Control, untreated hESCs; VE, VEGF-A; bFGF, bFGF; Vb, VEGF-A and bFGF; B4Vb, BMP4, VEGF-A and bFGF.
hESC-derived CD34+ progenitors can differentiate into functional endothelial cells in vitro and in vivo
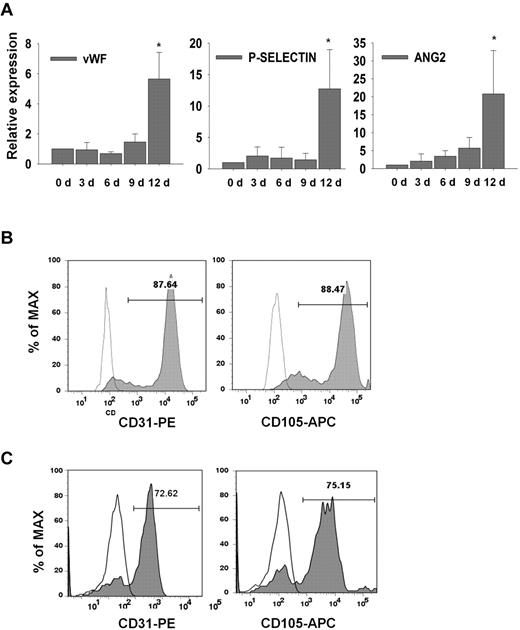
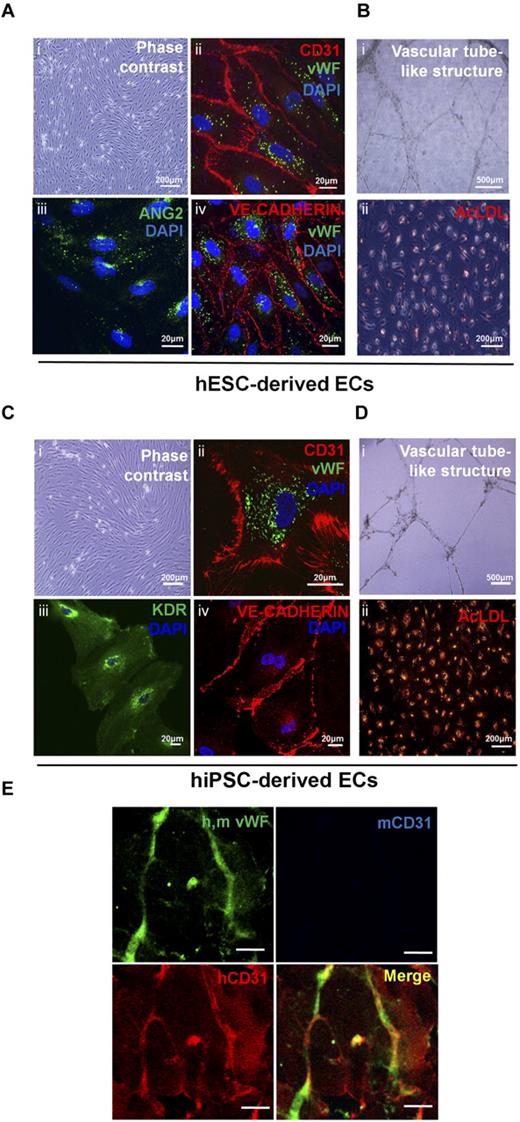
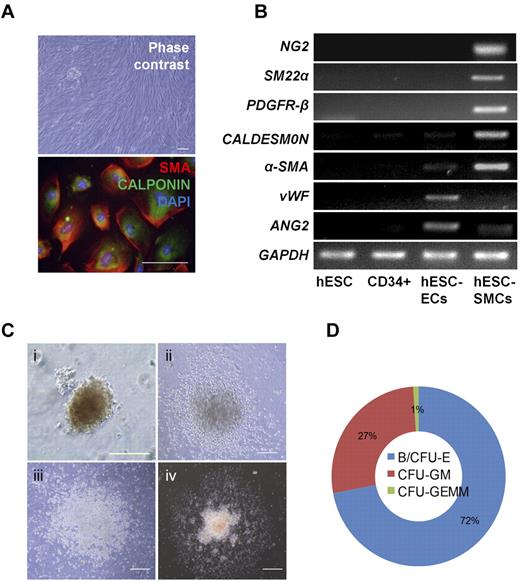
Vascular-lineage cells such as endothelial cells (ECs) and smooth muscle cells (SMCs) are derived from vascular progenitors and cardiovascular progenitors.17,29 In this study, hESC-derived CD34+cells were cultured in EGM-2 supplemented with VEGF-A and bFGF to induce differentiation into ECs. Mature EC marker genes (P-SELECTIN, VWF, and ANG2) were highly expressed at day 12 of culture (Figure 3A). Thus, it is likely that hESC-derived CD34+cells are vascular lineage progenitors. FACS analysis showed that a higher proportion of hPSC-derived CD34+cells could differentiate into ECs expressing endothelial markers such as CD31 and CD105 (Figure 3B,C). The ECs derived from hESCs and hiPSCs showed typical morphologies (Figure 4A,Ci), and strongly expressed endothelial cell-specific proteins, including CD31, VWF, VE-cadherin, ANG2, and KDR (Figure 4A-C). Furthermore, these cells formed vascular-like structures on Matrigel (Figure 4B-D, I) and took up acetylated-LDL (Figure 4B-Dii). To determine whether hESC-derived CD34+ cells can form blood vessels in vivo, 1 × 106 cells of hESC-derived CD34+ cells were mixed with Matrigel, and the mixture was then subcutaneously injected into a nude mouse. Human-specific CD31 positive signals were observed in the vessel-like structure at 10 days after injection of hESC-derived CD34+ cells, whereas mouse-specific CD31 positive signals were not detected (Figure 4E). Our results indicate that hESC-derived CD34+ progenitor cells have vasculogenic potential in vivo as well as in vitro. The hESC-derived CD34+ cells could also differentiate to SMCs when they were cultured in EGM-2 supplemented with platelet-derived growth factor-BB (PDGF-BB) and bFGF for 15 to 21 days. hESC-derived SMCs showed spindle-like morphologies and coexpressed α-SMA and CALPONIN in their cytoplasm (Figure 5A). They also expressed SMC marker genes such as NG2, SM22α, PDGFR-β, CALDESMON, and α-SMA, whereas the endothelial marker genes (VWF and Ang2) did not (Figure 5B). In addition, hESC-derived SMCs were contracted within 30 minutes after treatment with carbachol, an agonist against acetylcholine receptors (supplemental Video 1), but hESC-derived ECs were not (supplemental Video 2), indicating functionality of hESC-derived SMCs.
Expression of mature endothelial cell markers. (A) Relative expression of endothelial cell marker genes (VWF, P-SELECTIN, and ANG2). The samples were obtained at 3, 6, 9, 12 days of culture. The values are the mean ± SD of 3 independent experiments. A P value < .05 was considered to be statistically significant (*P < .05; n = 3). (B-C) Flow cytometric analysis for the endothelial markers CD31 and CD105 in hESC-derived (B) and hiPSC-derived (C) ECs.
Expression of mature endothelial cell markers. (A) Relative expression of endothelial cell marker genes (VWF, P-SELECTIN, and ANG2). The samples were obtained at 3, 6, 9, 12 days of culture. The values are the mean ± SD of 3 independent experiments. A P value < .05 was considered to be statistically significant (*P < .05; n = 3). (B-C) Flow cytometric analysis for the endothelial markers CD31 and CD105 in hESC-derived (B) and hiPSC-derived (C) ECs.
Endothelial cells from hESC-derived CD34+ progenitor cells are functional in vitro and in vivo. (A,C) Immunostaining for EC markers (CD31, VWF, KDR, ANG2, and VE-cadherin) in hESC- and hiPSC-derived ECs. hESC-derived ECs showed typical endothelial cell morphology (A and Ci), and expressed multiple EC markers (A and Cii-iv). (B) and (D) In vitro functional assay for hESC- and hiPSC-derived ECs. hESC- and hiPSC-derived ECs formed vascular tube-like structures on Matrigel (B and Di), and took up Dil-labeled acetylated-LDL (B,Dii). (E) Matrigel plug assay for hESC-derived CD34+ cells. Antibody for VWF was used to observe both mouse and human blood vessels. Species-specific CD31 antibodies were used to discriminate mouse and human blood vessels. Mouse and human specific CD31 antibodies are indicated as blue and red colors, respectively. Scale bar is 20 μm.
Endothelial cells from hESC-derived CD34+ progenitor cells are functional in vitro and in vivo. (A,C) Immunostaining for EC markers (CD31, VWF, KDR, ANG2, and VE-cadherin) in hESC- and hiPSC-derived ECs. hESC-derived ECs showed typical endothelial cell morphology (A and Ci), and expressed multiple EC markers (A and Cii-iv). (B) and (D) In vitro functional assay for hESC- and hiPSC-derived ECs. hESC- and hiPSC-derived ECs formed vascular tube-like structures on Matrigel (B and Di), and took up Dil-labeled acetylated-LDL (B,Dii). (E) Matrigel plug assay for hESC-derived CD34+ cells. Antibody for VWF was used to observe both mouse and human blood vessels. Species-specific CD31 antibodies were used to discriminate mouse and human blood vessels. Mouse and human specific CD31 antibodies are indicated as blue and red colors, respectively. Scale bar is 20 μm.
Differentiation of hESC-derived CD34+ cells to vascular smooth muscle cells and hematopoietic cells. (A) Morphology and expression of SMC markers in hESC-derived SMCs (α-SMA and CALPONIN). Cell nuclei were stained with DAPI (blue). Scale bar is 100 μm. B. Transcriptional expression of SMC (NG2, SM22α, PDGFR-β, CALDESMON, and α-SMA) and EC (VWF and ANG2) marker genes. (C) Various hematopoietic colonies were formed from hESC-derived CD34+ cells in methylcellulose medium: (i) BFU-E, (ii) CFU-GM, (iii) CFU-G, and (iv) CFU-GEMM. Scale bar is 100 μm. (D) The proportion of various hematopoietic cell-type colonies in CD34+ cells cultured in MethoCult.
Differentiation of hESC-derived CD34+ cells to vascular smooth muscle cells and hematopoietic cells. (A) Morphology and expression of SMC markers in hESC-derived SMCs (α-SMA and CALPONIN). Cell nuclei were stained with DAPI (blue). Scale bar is 100 μm. B. Transcriptional expression of SMC (NG2, SM22α, PDGFR-β, CALDESMON, and α-SMA) and EC (VWF and ANG2) marker genes. (C) Various hematopoietic colonies were formed from hESC-derived CD34+ cells in methylcellulose medium: (i) BFU-E, (ii) CFU-GM, (iii) CFU-G, and (iv) CFU-GEMM. Scale bar is 100 μm. (D) The proportion of various hematopoietic cell-type colonies in CD34+ cells cultured in MethoCult.
In the next experiment, a CFU assay was used to determine the differentiation potential of hESC-derived CD34+ cells to hematopoietic lineages. Various hematopoietic cells, including granulocytes, granulomacrophages, erythroids, and megakaryocytes, could be differentiated from hESC-derived CD34+ cells (Figure 5C), but not from CD34− cells. The CD34+ cells began to form colonies of erythroid-lineage cells, CFU-E (CFU-erythroid) at 6 days of culture in methylcellulose medium. Around 11 days of culture, erythroid-lineage cells such as BFU-E (burst-forming unit-erythroid) reached a maximum population (Figure 5C, I). Myeloid-lineage colonies, including CFU-GM (CFU-granulocyte, macrophage), CFU-G (CFU-granulocyte), and CFU-GEMM (CFU-granulocyte, erythroid, macrophage, megakaryocyte) were also observed between 14 and 21 days after plating on methylcellulose medium (Figure 5C, II, III, and IV). Hematopoietic lineage colonies were formed at an average of 200 CFUs per 105 CD34+ cells. Of these colonies, erythroid-lineage cells (eg, BFU-E and CFU-E) were abundant (Figure 5D). Collectively, hESC-derived CD34+ cells are bipotent in that they could differentiate into vascular lineage cells and various hematopoietic cells.
hESC-derived CD34+ progenitor cells improve perfusion and subsequent prognosis of ischemic mouse hind limbs by contributing to neovasculogenesis
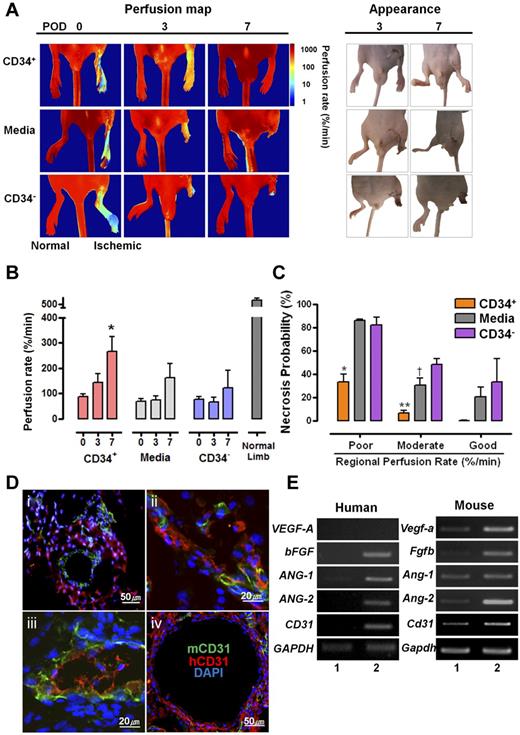
To determine the functionality of hESC-derived cells in vivo, approximately 1 × 106 hESC-derived CD34+ cells were intramuscularly injected into an ischemic hind limb. A total of 23 mice were divided into 3 groups; 9 mice were injected with CD34+ cells, 9 with medium alone, and 5 with CD34− cells. The initial perfusion rates of the ischemic limbs were not significantly different between groups (ANOVA test, F2,20 = .761, P = .48). After injection of hESC-derived CD34+ cells into ischemic limbs, follow-up indocyanine green perfusion imaging was subsequently performed on POD 3 and 7. As shown in the perfusion map in Figure 6A, mouse ischemic hind limbs with hESC-derived CD34+ cells were recovered at POD 3 and further clearly at POD7, whereas those injected with the medium or hESC-derived CD34− cells resulted in necrosis at the end of the hind limbs. These differences between the groups were visually obviously (Figure 6A photographs in “appearance” panel). Intergroup comparisons of time-dependent increments in the perfusion rates of the ischemic limbs clearly demonstrated the therapeutic effects of CD34+ cells, especially on POD 7 (Figure 6B). The probability of necrosis of the ischemic region was estimated by the relationship between the probability of regional tissue necrosis on POD 7 and the initial tissue perfusion rate/measured perfusion rate of the corresponding region estimated immediately after surgery. In the hESC-derived CD34+ cells-injected group, regions with poor and moderate perfusion rate ranging lower than 120%/min, which is approximately 20% of the perfusion rate of normal tissue, showed statistically significant decreases of necrosis probability compared with that of media or hESC-derived CD34− cells-injected group (Figure 6C). To investigate how transplanted hESC-derived CD34+cells recover the ischemic hind limb, tissues of the operated region were harvested on POD 7, and the presence of hESC-derived ECs was assessed by immunostaining using antibodies specific for human CD31. The coexistence of human and mouse CD31+ cells was observed in ischemic hind-limb regions injected with hESC-derived CD34+ cells. This implies that hESC-derived CD34+ progenitor cells can develop into ECs in vivo, thereby participating in neovasculogenesis of the ischemic hind-limb region. In fact, hESC-derived CD34+ cells are likely to contribute to neovasculogenesis in the ischemic region within a week after injection. In most cases, human CD31+ cells were localized outside of the mouse blood vessels (Figure 6Di) and inserted in part into hybrid blood vessels with mouse CD31+ cells (Figure 6Dii-iii). Rarely, blood vessels that were mainly composed of human CD31+ cells were also observed. (Figure 6Div). When 3 sections of the ischemic tissue were randomly taken and then examined for the contribution states, proportion of outside (Figure 6Di), hybrid (Figure 6Dii-iii), and whole (Figure 6Div) types was 45.5% (30/66 cases), 51.5% (34/66 cases), and 3% (2/66 cases), respectively. In addition to direct contribution of human CD31+ cells in neovasculargenesis, whether the injected hESC-derived CD34+ cells can promote mouse angiogenesis indirectly, we addressed transcriptional expressions of angiogenesis-related genes such as VEGF-A, bFGF, ANG1, and ANG2 were examined from the ischemic tissue sample. As shown in Figure 6E, the human angiogenesis-related genes such as bFGF, ANG-1, and ANG-2 were expressed in the ischemic tissue injected with hESC-derived CD34+ cells. Interestingly, mouse angiogenesis-related genes such as Vegfa, Fbfb, and Ang-2 were also highly expressed in the ischemic tissue. These data imply that angiogenic cytokines secreted from hESC-derived CD34+ cells may indirectly contribute to neovasculogenesis in the ischemic hind limbs. In addition, this study demonstrates that hiPSC-derived CD34+ cells can improve salvage of ischemic hind limbs (supplemental Figure 6). Like hESCs, ischemic hind limbs injected with hiPSC-derived CD34+ cells were recovered by POD 7 (supplemental Figure 6A). The coexistence (yellow arrowhead) of human CD31+ cells and mouse lectin+CD31+ cells was observed in ischemic hind-limb regions injected with hiPSC-derived CD34+ cells, representing functional blood vessels (supplemental Figure 6B). Another type of hiPSC-derived CD31+ cells surrounding the mouse blood vessels was detected (supplemental Figure 6B). Necrosis probability of ischemic hind limbs injected with hiPSC-derived CD34+ cells at POD 7 was diminished in the ischemic regions in a manner similar to that of hESC derivatives (supplemental Figure 6C). These observations indicate that hPSC-derived CD34+ progenitor cells are involved, either directly or indirectly, in neovasculogenesis, causing functionality in the ischemic model.
Therapeutic effects of hESC-derived CD34+ progenitor cells on neovasculogenesis in ischemic mouse hind limbs. (A) Comparison of perfusion rates and prognosis in ischemic limbs injected with CD34+, medium, or CD34− cells. Left panel indicates indocyanine green perfusion maps obtained at 0, 3, and 7 days after surgery. The perfusion maps of day 0 show tissue perfusion distribution of the entire lower half of the body, including normal limbs. Right panel shows photographs of the hind limbs at 3 and 7 days after injection. (B) Average perfusion rates of ischemic hind limbs according to POD are indicated for each group. *P = .014 vs POD 0 (ANOVA F2,24 = 5.311, P = .012). (C) Probability of necrosis; the relationship between the probability of regional tissue necrosis on POD 7 and the tissue-perfusion rate of the corresponding region estimated immediately after surgery. The X axis shows the regional perfusion rate; poor (lower than 15%/min), moderate (16%-120%/min), and (> 120%/min). ANOVA and Scheffe post-hoc test applied to the significant effect of groups on poor and moderate perfusion rate, (ANOVA F2,3 = 27.993, P = .011. *P = .015 vs media-treated group and P = .02 vs SC34− cells-treated group with poor perfusion rate; ANOVA F2,9 = 18.872, P = .001. **P = .02 vs media-treated group and P = .001 vs SC34− cells-treated group with a moderate perfusion rate. (D) Various types of hESC-derived CD34+ cells involved in neovasculogenesis in ischemic hind limbs. hESC-derived CD34+ cells could contribute indirectly (i), partially (ii-iii), or mainly (iv) to neovasculogenesis. Immunohistochemical analysis of ischemic hindlimb regions transplanted with hESC-derived CD34+ cells. (E) Expression of angiogenic genes in the ischemic region injected with hESC-derived CD34+ cells. Human- and mouse-specific primers were used for analyzing the expression of various angiogenic genes: (1) normal hind limb tissue, (2) ischemic hind-limb tissue injected with the hESC-derived CD34+ cells.
Therapeutic effects of hESC-derived CD34+ progenitor cells on neovasculogenesis in ischemic mouse hind limbs. (A) Comparison of perfusion rates and prognosis in ischemic limbs injected with CD34+, medium, or CD34− cells. Left panel indicates indocyanine green perfusion maps obtained at 0, 3, and 7 days after surgery. The perfusion maps of day 0 show tissue perfusion distribution of the entire lower half of the body, including normal limbs. Right panel shows photographs of the hind limbs at 3 and 7 days after injection. (B) Average perfusion rates of ischemic hind limbs according to POD are indicated for each group. *P = .014 vs POD 0 (ANOVA F2,24 = 5.311, P = .012). (C) Probability of necrosis; the relationship between the probability of regional tissue necrosis on POD 7 and the tissue-perfusion rate of the corresponding region estimated immediately after surgery. The X axis shows the regional perfusion rate; poor (lower than 15%/min), moderate (16%-120%/min), and (> 120%/min). ANOVA and Scheffe post-hoc test applied to the significant effect of groups on poor and moderate perfusion rate, (ANOVA F2,3 = 27.993, P = .011. *P = .015 vs media-treated group and P = .02 vs SC34− cells-treated group with poor perfusion rate; ANOVA F2,9 = 18.872, P = .001. **P = .02 vs media-treated group and P = .001 vs SC34− cells-treated group with a moderate perfusion rate. (D) Various types of hESC-derived CD34+ cells involved in neovasculogenesis in ischemic hind limbs. hESC-derived CD34+ cells could contribute indirectly (i), partially (ii-iii), or mainly (iv) to neovasculogenesis. Immunohistochemical analysis of ischemic hindlimb regions transplanted with hESC-derived CD34+ cells. (E) Expression of angiogenic genes in the ischemic region injected with hESC-derived CD34+ cells. Human- and mouse-specific primers were used for analyzing the expression of various angiogenic genes: (1) normal hind limb tissue, (2) ischemic hind-limb tissue injected with the hESC-derived CD34+ cells.
Discussion
Developing a simple and efficient protocol for the differentiation of hPSCs into a specialized cell type is a key factor for future cell therapy. Reprogramming of human somatic cells to the pluripotent state could also provide a breakthrough for clinical applications in cell therapy using pluripotent stem cells.30 In this study, an efficient method for directly differentiating hESCs and hiPSCs into functional CD34+ progenitor cells was achieved by both inhibition of MEK/ERK signaling and activation of BMP4 signaling. To date, vascular lineage cells have usually been isolated from spontaneously differentiated cells via formation of embryoid bodies or by coculturing with mouse stromal cells in hESCs and hiPSCs.17,23,31-36 In the present study, vascular lineage cells could be differentiated directly from hESC-derived CD34+ progenitors. ECs originating from hPSC-derived CD34+ progenitors could form vascular tube-like structure in vitro and de novo blood vessels in vivo (Figure 4). Thus, it is likely that the hPSC-derived CD34+ progenitors may differentiate into vascular lineage cells with functionality. It has been known that CD34+ progenitor cells isolated from peripheral blood, cord blood, and bone marrow of animals and human have therapeutic potential for cardiovascular diseases such as ischemia and myocardial infarction.21,37-39 As an alternative approach, therapeutic cells can be generated from pluripotent stem cells such as hESCs and hiPSCs. It has been reported that hESC-derived endothelial cells are effective for the restoration of the ischemic hind limb.24 Ischemic hind limbs injected with hPSC-derived ECs could be salvaged around 4 weeks after injection, representing a longer period of therapeutic effect. VEGFR2+TRA-1-60− vascular progenitor cells derived from hESCs could be also differentiated into endothelial and smooth muscle cells.23 These VEGFR2+TRA-1-60− vascular progenitor cells were not effective in recovering the ischemic hind limb, although the vascular progenitor cell-derived ECs could salvage the ischemic hind limb 9 days after injection into the femoral artery.23 In contrast, we showed that hPSC-derived CD34+ progenitor cells recovered ischemic hind limbs within only a week of injection of the CD34+ progenitors (Figure 6 and supplemental Figure 6). Furthermore, hPSC-derived ECs were incorporated into mouse blood vessels (Figure 6D and supplemental Figure 6B), indicating that hPSC-derived CD34+ cells may directly contribute to the neovasculogenesis in the ischemic hind limb. Nonetheless, another result (Figure 6E) offers a hint that angiogenic factors secreted from hESC-derived ECs may facilitate the formation of mouse blood vessels in ischemic hind limbs. Collectively, our results demonstrate that hPSC-derived CD34+ progenitors may be more effective in curing ischemic disease than mature endothelial cells.
Apart from vasculogenic competence of hESC-derived ECs, hESC-derived CD34+ cells can differentiate into a variety of hematopoietic cells in vitro.40,41 However, transplanted hESC-derived CD34+ cells or CD34+CD38− have limitations for engrafting into bone marrow in NOD/SCID/γc−/− mice and fetal sheep, respectively.42,43 In contrast to hESC-derived CD34+ cells, human CD34+ cells originated from umbilical cord blood could contribute to long-term bone marrow engraftment in NOD/SCID/IL-2Rc2/2 mice.44 This functional discrepancy between hESC- and umbilical cord blood-derived CD34+ cells may be explained by their phenotypic differences. Unlike umbilical cord blood-derived CD34+ cells, hESC-derived CD34+ cells express CD31, CD90, CD73, and FLK1, but not CD45 and CD117.43 Similarly, hESC-derived CD34+ cells generated in the present study coexpressed CD31, CD90, and CD73, but did not express CD45 (supplemental Figure 7A). In addition, only a small proportion (5%) of hESC-derived CD34+ cells expressed CD43, an early hematopoietic progenitor marker (supplemental Figure 7B). Because of both phenotypic differences and a paucity of hematopoietic progenitors, hESC-derived CD34+ cells probably have limitations for bone marrow engraftment.
It has been reported that overall gene expression profiles of hiPSCs are similar to those of hESCs.9,10 In our study, however, a few differences were observed in the differentiation processes between hESCs and hiPSCs. First, responses to chemicals or growth factors appeared to be slightly different. For induction to the mesodermal lineage, hESCs were treated with MEK inhibitor and BMP4 for 3 days, while hiPSCs were treated for 5 days; for subsequent differentiation into CD34+ cells, it took 6 days in hESCs and 9 days in hiPSCs (supplemental Figure 3). Secondly, differentiation potentials into a specialized cell types seem to be distinct; unlike hESC-derived CD34+ cells, hiPSC-derived CD34+ cells could not form hematopoietic colonies (supplemental Figure 8A), and the resultant cells did not express hematopoietic genes (supplemental Figure 8B). Therefore, it is conceivable that hiPSC-derived CD34+ cells are likely to develop into endothelial cells, not hematopoietic cells. To clarify this biased differentiation potential, further experiments should be done in several hiPSC lines.
The method for direct differentiation developed in this study has several advantages for inducing hPSCs to specialized cell types. First, inhibition of MEK/ERK signaling and activation of BMP4 signaling synergistically trigger the efficient production of mesoderm-lineage cells (Figure 1). hESCs could be induced to differentiate to extra-embryonic lineages without apoptosis by inhibition of MEK/ERK signaling.3 BMP4 could trigger the differentiation of hESCs to trophoblasts or primitive endoderm,11,12 whereas BMP4 treatment could facilitate mesoderm differentiation.13 In this study, we showed that simultaneous inhibition of MEK/ERK signaling plus activation of BMP4 signaling synergistically facilitated the induction of hESCs and hiPSCs to mesoderm-lineage cells. Second, the feeder- and serum-free systems used herein for hPSC differentiation will be useful for the manipulation of hPSCs destined for cell therapy. During our differentiation process from hESCs and hiPSCs to CD34+ progenitor cells, animal supplements (eg, mouse embryonic fibroblast [MEF] or FBS) were not added to the differentiation medium. The use of chemical agents versus animal-derived factors is likely to be safer for inducing differentiation of hPSCs intended for cell therapy. In addition, our chemical-based, feeder-free system will be helpful for studying the mechanisms of hPSC differentiation, because the side effects from unknown (i.e., animal-derived) factors will be minimized. Third, our stepwise protocol for differentiation of hPSCs may be useful for studies aimed at exploring the properties of lineage-specific progenitors and understanding the differentiation process of stem cells or progenitors to specialized cell types. In the present study, mesoderm-lineage cells were first induced from hPSCs by PD98059 and BMP4. Then, a high proportion of CD34+ progenitor cells were derived from mesoderm-lineage cells cultured in medium supplemented with VEGF-A and bFGF. Finally, CD34+ progenitor cells were differentiated into vascular and hematopoietic cells. The specialized cell types generated in each experimental step could be helpful for studying vasculogenesis and hematopoiesis in vitro.
In summary, this study demonstrates that functional CD34+ progenitor cells could be efficiently differentiated from hESCs and hiPSCs in animal serum- and feeder-free systems by combined the regulation of 2 signaling pathways. In addition, it is suggested that the hPSC-derived CD34+ progenitor cells produced by this novel method have the potential to cure vascular diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr K. Jung for providing recombinant human VEGF-A. This research was supported by a grant (no. SC-2210) from the Stem Cell Research Center, a grant (no. 2009-0084073) from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST); and a grant (no. A084697) from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea.
Authorship
Contribution: S.-W.P. and Y.-M.H designed and organized the experiments, analyzed data, and wrote the manuscript; S.-W.P. and Y.-H.C. performed differentiation experiments to vascular and hematopoietic lineages, respectively; M.-J.J. maintained hESCs and hiPSCs; Y.J.K. and G.Y.K. contributed to immunohistochemistry analysis and Matrigel plug assay; Y.K., J.J., and C.C. carried out in vivo functionality of hESC-derived cells in hind-limb ischemic mice via near-infrared fluorescence imaging; and M.-J.K., Y.S.C., and H.-M.C. generated and characterized hiPSCs and CHA4-hES.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong-Mahn Han, Department of Biological Sciences, KAIST, 335 Gwahangno Yuseong-gu, Daejeon 305-701, Republic of Korea; e-mail: ymhan@kaist.ac.kr.