Abstract
The Southwest Oncology Group conducted a randomized trial comparing lenalidomide (LEN) plus dexamethasone (DEX; n = 97) to placebo (PLC) plus DEX (n = 95) in newly diagnosed myeloma. Three 35-day induction cycles applied DEX 40 mg/day on days 1 to 4, 9 to 12, and 17 to 20 together with LEN 25 mg/day for 28 days or PLC. Monthly maintenance used DEX 40 mg/day on days 1 to 4 and 15 to 18 along with LEN 25 mg/day for 21 days or PLC. Crossover from PLC-DEX to LEN-DEX was encouraged on progression. One-year progression-free survival, overall response rate, and very good partial response rate were superior with LEN-DEX (78% vs 52%, P = .002; 78% vs 48%, P < .001; 63% vs 16%, P < .001), whereas 1-year overall survival was similar (94% vs 88%; P = .25). Toxicities were more pronounced with LEN-DEX (neutropenia grade 3 or 4: 21% vs 5%, P < .001; thromboembolic events despite aspirin prophylaxis: 23.5% [initial LEN-DEX or crossover] vs 5%; P < .001). This trial was registered at www.clinicaltrials.gov as #NCT00064038.
Introduction
The immunomodulatory agent lenalidomide (LEN) was approved by the U.S. Food and Drug Administration for use in combination with dexamethasone (DEX) for previously treated myeloma. LEN directly induces myeloma cell apoptosis and exerts indirect effects via modulation of the bone marrow microenvironment and immune system.1,2 Results of a multicenter randomized placebo-controlled trial S0232 by the Southwest Oncology Group (SWOG) demonstrated superiority of LEN-DEX over placebo (PLC)–DEX as first-line therapy for multiple myeloma patients.
Methods
Untreated myeloma patients were randomized to either LEN-DEX or PLC-DEX, using a dynamic balancing algorithm to assign treatment.3 Initial randomization was stratified by International Staging System stage4 (1 vs 2 vs 3) and Zubrod performance status (0 or 1 vs 2 or 3). Transplantation-ineligible or -denying patients had to have symptomatic disease with a measurable M-protein, be at least 18 years old, and have a performance status less than 3 (unless resulting from myeloma). Induction therapy consisted of 3 35-day cycles of DEX 40 mg/day on days 1 to 4, 9 to 12, and 17 to 20 plus LEN at 25 mg/day for 28 days or PLC. Maintenance therapy consisted of DEX 40 mg/day on days 1 to 4 and 15 to 18 plus LEN 25 mg/day for 21 days or PLC in repeating 28-day cycles. Both treatment arms were continued until disease progression or unacceptable toxicity. After a high incidence of thromboembolic events (TEEs) in the first 21 patients, all subsequent patients were required to take aspirin 325 mg/day, unless already on anticoagulation therapy for other reasons. On disease progression, patients on PLC-DEX could cross over to open-label LEN-DEX. Participants with progressive disease after therapy with LEN-DEX (initially or after crossover) were removed from the protocol.
The primary objective was to compare progression-free survival (PFS), defined as the time from registration until either progressive disease or death from any cause, between the 2 treatment groups. Other objectives included comparisons of overall response rate5,6 overall survival (OS), and toxicity. The original study design called for accrual of 500 patients, to have 83% power to detect a 33% improvement in PFS on the experimental arm. PFS and OS were estimated by the Kaplan-Meier method7 and compared on an intent-to-treat basis using the log-rank test. Response rates according to both SWOG and IMWG criteria were compared using χ2 tests. Patients who did not progress or died were censored at the date of last contact for this analysis.
Results and discussion
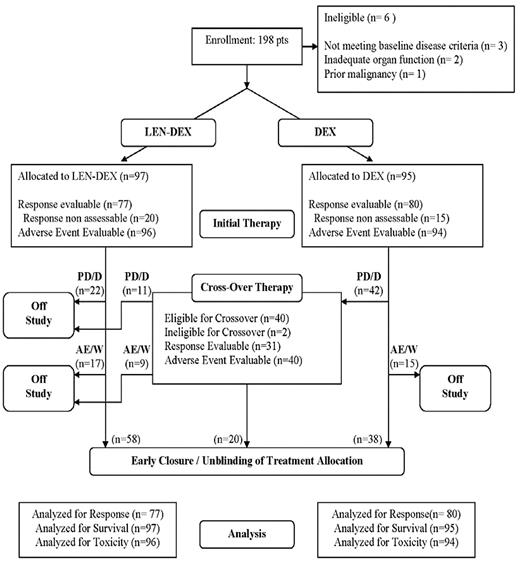
Between October 15, 2004 and April 2, 2007, 198 patients were enrolled. Based on inferior efficacy of PLC-DEX8 and concern for the relative safety of combining LEN with DEX in excess of 40 mg weekly,9 the Data and Safety Monitoring Committee recommended early study closure. Original treatment allocation was unblinded, open-label LEN-DEX was made available to all patients, and dose reduction of DEX to 40 mg weekly was recommended. The flow of all registered patients is summarized in Figure 1.
Trial design and patient flow. PD/D indicates progressive disease or death; and AE/W, adverse event or withdrawal.
Trial design and patient flow. PD/D indicates progressive disease or death; and AE/W, adverse event or withdrawal.
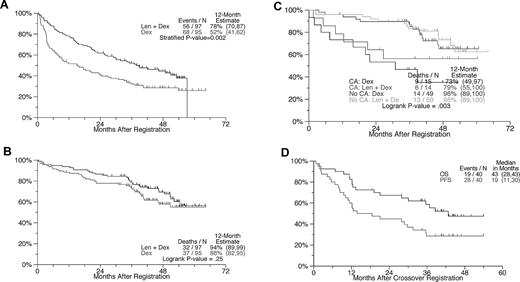
Initial therapy
Baseline characteristics were well matched between arms (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cytogenetic abnormalities (CAs) were present in 29 of 128 patients. As of July 16, 2010, the median follow-up of live patients was 47.2 months. The 1-year PFS with LEN-DEX was superior at 78% compared with 52% for the control arm (Figure 2A, P = .002). At 3 years, PFS remained superior for LEN-DEX: 52% vs 32%. Regardless of randomization, PFS was significantly shorter in patients with than without CAs (both P < .05), supporting Mayo Clinic data.10 Overall response (partial response or better) was higher with LEN-DEX (78% vs 48%; P < .0001) as was the very good partial response rate (63% vs 16%; P < .001; supplemental Table 2). The superior response rate of LEN-DEX compared with DEX is consistent with data from other studies in the frontline11-13 and relapsed/refractory settings.14,15 In addition, although the overall response rate achieved in S0232 is similar to that reported with thalidomide plus dexamethasone (THAL-DEX) in 2 large trials,8,16 the superior very good partial response rate and PFS achieved with LEN-DEX underscore its greater efficacy compared with THAL-DEX as initial therapy of multiple myeloma.11,17
PFS and OS from the times of randomization and crossover with attention to the effects of cytogenetic abnormalities. (A) PFS: Estimated 1-year PFS rate was 78% with LEN + DEX and 52% with DEX (P = .0003). (B) OS: No difference is apparent between arms. (C) OS according to the presence or absence of CAs: patients without CAs had superior OS, regardless of treatment arm. No statistically significant OS differences between LEN-DEX and DEX could be demonstrated within the CA and no CA groups. Testing CA versus No CA within treatment groups: LEN-DEX, P = .09; DEX, P = .03. Testing LEN-DEX versus DEX within CA groups: CA, P = .42; No CA, P = .7. (D) PFS and OS after crossover from DEX to LEN-DEX.
PFS and OS from the times of randomization and crossover with attention to the effects of cytogenetic abnormalities. (A) PFS: Estimated 1-year PFS rate was 78% with LEN + DEX and 52% with DEX (P = .0003). (B) OS: No difference is apparent between arms. (C) OS according to the presence or absence of CAs: patients without CAs had superior OS, regardless of treatment arm. No statistically significant OS differences between LEN-DEX and DEX could be demonstrated within the CA and no CA groups. Testing CA versus No CA within treatment groups: LEN-DEX, P = .09; DEX, P = .03. Testing LEN-DEX versus DEX within CA groups: CA, P = .42; No CA, P = .7. (D) PFS and OS after crossover from DEX to LEN-DEX.
OS was not different between arms in S0232 (1-, 2-, and 3-year OS values for LEN-DEX were 94%, 87%, 79% vs 88%, 78%, 73%, respectively, for PLC-DEX; P = .28; Figure 2B). This can be explained by the crossover design, the availability of other salvage therapies, and the impact early study closure had on statistical power. Early deaths (defined as death before the first response assessment at 6 weeks) occurred in 1% of patients on LEN-DEX and 3% of those on PLC-DEX. The presence of CAs imparted shorter OS in both study arms (Figure 2C).
Efficacy of crossover therapy
Of 40 eligible patients crossing over from PLC-DEX to LEN-DEX before study closure and unblinding of original treatment allocation, all were assessable for toxicity and 31 were evaluable for response. The median PFS from the time of crossover was 19 months, and the median OS was approximately 43 months (Figure 2D), consistent with experience in relapsed myeloma.14,15
Safety
Myelosuppression was more frequent with LEN-DEX (grades 3 or 4: 21% vs 5%; P = .0009; supplemental Table 3A), possibly explaining the higher rate of infection (supplemental Table 3B). The frequency of adverse events after crossover from PLC-DEX to LEN-DEX was similar to that seen in patients initially randomized to LEN-DEX induction (supplemental Table 3C). The types of adverse events experienced by patients treated with LEN-DEX in our study were similar to those reported previously.14,15,18 Consistent with previous reports, TEE occurred more often with LEN-DEX.19-21 Before instituting aspirin thromboprophylaxis, TEE occurred in 8 of the first 12 patients randomized to LEN-DEX versus none in the control arm (P = .0019). Our previous findings of TEE reduction by aspirin prophylaxis22 were not confirmed as a TEE excess with LEN-DEX persisted after amendment (19% vs 6%; P = .0095). Therefore, other thromboprophylaxis strategies, such as low molecular weight heparin, should be considered, particularly if any other risk factors are present or DEX dosing exceeds 40 mg/week.23
In conclusion, S0232 confirms the superiority LEN-DEX over PLC-DEX alone as first-line therapy in terms of response rates and PFS. The higher incidence of TEE despite aspirin 325 mg prophylaxis calls attention to the need for more effective thromboprophylaxis. SWOG is currently leading a US Intergroup trial S0777 comparing LEN-DEX versus LEN-DEX plus bortezomib as initial myeloma treatment, using lower DEX dosing (40 mg/week) to minimize glucocorticoid-associated toxicity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marjorie A. Godfrey, Dana Sparks, and Harry Erba (SWOG), and Robert Knight and Jerome Zeldis (Celgene Corporation).
This work was supported in part by the National Cancer Institute, Department of Health and Human Services (Public Health Service Cooperative Agreement grants CA32102, CA38926, CA04919, CA35431, CA14028, CA67575, CA35090, CA58861, CA20319, CA35178, CA76429, CA35176, CA68183, CA95860, CA27057, CA35119, CA35281, CA45808, CA37981, CA16385, CA76448, CA63848, CA12644, CA86780, CA46282, CA58658, CA11083, CA58882, and CA58416) and by Celgene Corporation.
National Institutes of Health
Authorship
Contribution: B.B., J.C., B.G.M.D., M.A.H., and J.A.Z. conceived and designed the study; B.B., V.B., J.C., B.G.M.D., and J.A.Z. analyzed and interpreted data; V.B. collected and assembled data; J.A.Z. and B.B. wrote the manuscript; M.H.A. provided administrative support (responded to study inquiries in the absence of the study coordinator); M.H.A., B.B., V.B., B.G.M.D., J.C., M.A.H., D.F.M., B.F.W., and J.A.Z. reviewed and approved the manuscript; and B.B., M.A.H., D.F.M., B.F.W., and J.A.Z. provided study materials/patients.
Conflict-of-interest disclosure: B.B. received research funding and was on the speaker's bureau for Celgene and Millennium and received honoraria from Celgene. B.G.M.D. received research funding and honoraria from Celgene. M.A.H. is presently employed by Celgene, but was not at the time the study was conducted. J.A.Z. received research funding and was on the speaker's bureau (continuing medical education only; no promotional speaking) for Celgene and Millennium. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey A. Zonder, Wayne State University, Karmanos Cancer Institute, Division of Hematology/Oncology, 4 HWCRC, 4100 John Rd, Detroit, MI 48201; e-mail: zonderj@karmanos.org.