To the editor:
Familial hemophagocytic lymphohistiocytosis (FHL) is a genetic disorder of lymphocyte cytotoxicity caused by mutations in the gene encoding perforin (FHL-2) or in genes encoding proteins important for intracellular trafficking and exocytosis of perforin-containing lytic granules.1 These include Munc13-4 (FHL-3), syntaxin 11 (FHL-4), and Munc18-2 (FHL-5). The molecular machinery for the transport of lytic granules in part overlaps with that required for the transport of lysosome-related organelles in other cellular systems. This is well illustrated by immunodeficiency syndromes associated with albinism such as Griscelli syndrome type II (GSII), Chediak-Higashi syndrome (CHS), and Hermansky-Pudlak syndrome type 2 (HPS2). The genes affected in these diseases (RAB27A, LYST, and AP3B1) are also relevant for hair pigmentation, neurologic development, neutrophil development, and platelet function leading to manifestations such as partial albinism, mental retardation, neutropenia, and bleeding in addition to a high risk of hemophagocytic lymphohistiocytosis (HLH).2,3
The recently described FHL-5 is considered to be mainly a disorder of cytotoxicity.4,5 A role for Munc18-2 has been demonstrated in mast cell degranulation,6 but a clinically relevant role for Munc18-2 deficiency in cell systems other than lymphocytes has not been documented. Munc18-2 is expressed in platelets and associates with a large complex containing synaptosome-associated protein of 23 kDa (SNAP-23) and cellubrevin/vesicle-associated membrane protein 3 (VAMP3).7 These data suggest that Munc18-2 might play a role in platelet granule exocytosis. In support of this concept, it was recently reported that 3 of 11 FHL-5 patients developed significant bleeding symptoms even outside of acute HLH episodes.8 However, all of these patients were also thrombocytopenic and platelet function tests were not performed.
Here we report on platelet function analysis of 4 patients with genetically confirmed FHL-5. The MUNC18-2 mutations of these patients, their clinical course, and data showing impaired T cell and NK cell degranulation have been described previously4,9 : patients P1155 and P19454 showed a typical course of FHL with manifestation of hemophagocytic syndrome within the first year of life, while patients P1 and P49 presented with a late onset of FHL. Of note, none of the patients had obvious bleeding symptoms.
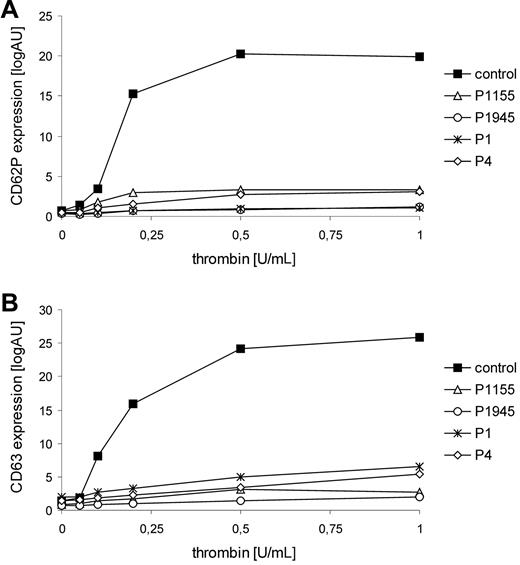
Flow cytometric analyses of the patients' platelets revealed that platelet α (CD 62P)– and δ (CD 63)–granule secretion in response to thrombin stimulation was severely impaired in all patients (Figure 1). Surface expression of glycoprotein (GP) Ib/V/IX and GPIIb/IIIa, ristocetin-induced binding of Von Willebrand factor, and binding of soluble fibrinogen were normal in all patients. Platelet aggregation/agglutination after stimulation with ADP, collagen, and ristocetin was slightly impaired. Bleeding time was assessed in P1945 and was slightly prolonged (8.5 minutes). These data demonstrate a selective impairment of platelet granule secretion in patients with FHL-5 and thus document an important role for Munc18-2 in platelet degranulation. Platelet secretion defects have also been observed in patients with HPS2 or with CHS who can present with mucocutaneous bleedings, especially after surgery.2,10 Although bleeding symptoms in FHL-5 patients seem to be mild, our findings clearly demonstrate that Munc18-2 deficiency is more than a genetic disorder of cytotoxicity.
Thrombin-induced granule secretion of FHL-5 platelets. Thrombin-mediated expression of CD62P (A) and CD63 (B) agonist on platelets was detected by flow cytometry. Diluted PRP (5 × 107/mL) was stimulated with different concentrations of thrombin (0.05-1.0 U/mL; Dade Behring) in the presence of 1.25mM Gly-Pro-Arg-Pro/GPRP. Platelets were stained by monoclonal anti-CD62P antibody (CLBthromb/6-FITC, Immunotech) and anti-CD63 antibody (CLB-gran/12-FITC), respectively. Data are expressed as median of logarithmic fluorescence intensities.
Thrombin-induced granule secretion of FHL-5 platelets. Thrombin-mediated expression of CD62P (A) and CD63 (B) agonist on platelets was detected by flow cytometry. Diluted PRP (5 × 107/mL) was stimulated with different concentrations of thrombin (0.05-1.0 U/mL; Dade Behring) in the presence of 1.25mM Gly-Pro-Arg-Pro/GPRP. Platelets were stained by monoclonal anti-CD62P antibody (CLBthromb/6-FITC, Immunotech) and anti-CD63 antibody (CLB-gran/12-FITC), respectively. Data are expressed as median of logarithmic fluorescence intensities.
Authorship
Acknowledgment: This work was supported by the German Federal Ministry of Education and Research (BMBF 01 EO 0803).
Contribution: L.N. performed research and analyzed data; K.S., S.E., and B.Z. designed research, analyzed data and wrote paper; and T.V. and K.B. took care of the patients. K.S. and L.N. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr Barbara Zieger, Department of Pediatrics and Adolescent Medicine, University of Freiburg, Mathildenstr 1, 79106 Freiburg, Germany; e-mail: barbara.zieger@uniklinik-freiburg.de.