Abstract
Treatment of hepatitis C (HCV)–mixed cryoglobulinemia (MC) may target either the viral trigger (HCV) or the downstream B-cell clonal expansion. Prospective cohort study of 38 HCV-MC patients who received a combination of rituximab (375 mg/m2) once a week for 1 month followed by Peg-interferon-α (Peg-IFN-α; 2a, 180 μg or 2b, 1.5 μg/kg) weekly plus ribavirin (600-1200 mg) daily for 48 weeks were compared with 55 HCV-MC patients treated by Peg-IFN-α/ribavirin with the same modalities. In the whole population of HCV-MC patients (n = 93), a complete clinical response was achieved in 73.1% (68 of 93), cryoglobulin clearance in 52.7% (49 of 93), and a sustained virologic response in 59.1% (55 of 93). Compared with Peg-IFN-α/ribavirin, rituximab plus Peg-IFN-α/ribavirin–treated patients had a shorter time to clinical remission (5.4 ± 4 vs 8.4 ± 4.7 months, P = .004), better renal response rates (80.9% vs 40% of complete response, P = .040), and higher rates of cryoglobulin clearance (68.4% vs 43.6%, P = .001) and clonal VH1-69+ B-cell suppression (P < .01). Treatment was well tolerated with 11% of discontinuation resulting from antiviral therapy and no worsening of HCV RNA under rituximab. Our findings indicate that rituximab combined with Peg-IFN-α/ribavirin is well tolerated and more effective than Peg-IFN-α/ribavirin in HCV-MC.
MedscapeCME Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 0.75 AMA PRA Category 1 credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/journal/blood; and (4) view/print certificate. For CME questions, see page 504.
Disclosures
The authors and Associate Editor A. Keith Stewart declare no competing financial interests. The CME questions author Désirée Lie, University of California, Irvine, CA, served as a nonproduct speaker for “Topics in Health” for Merck Speaker Services.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe clinical and immunologic viral manifestations of HCV-related mixed cryoglobulinemia (MC)
Define complete, partial, and nonresponse to treatment of HCV-related MC
Describe immunologic responses and predictors of response to treatment with peg-interferon α/ribavirin with and without rituximab in HCV patients with MC
Introduction
Mixed cryoglobulinemia (MC) is a systemic vasculitis that mainly affects the small-sized and, less frequently, medium-sized vessels.1 MC reflects the expansion of B cells producing a pathogenic IgM with rheumatoid factor activity.2 MC leads to clinical manifestations ranging from the so-called MC syndrome (purpura, arthralgia, and asthenia) to more serious lesions with neurologic and renal involvement.3 With the discovery of HCV as the etiologic agent for most cases of mixed cryoglobulinemia, new opportunities and problems for crafting therapy of HCV-MC have emerged. A new and major concern was the potential adverse effects that immunosuppressive therapy with glucocorticoids and cytotoxic drugs could have on an underlying chronic viral infection. Alternatively, the discovery of HCV provided the opportunity to control HCV-MC with antiviral therapy based on the belief that the underlying infection was driving immune complex formation and resultant vasculitis.4
Treatment of hepatitis C (HCV)-MC with severe organ involvement remains difficult and may target either the viral trigger (HCV) or the downstream B-cell arm of autoimmunity.5-8 Inducing a sustained virologic and clinical response and minimizing the use of immunosuppressive drugs are the main goals in the treatment of patients with HCV-MC vasculitis. Antiviral therapy has been shown to reverse bone marrow monoclonal B-cell expansion in patients with HCV-MC.9 Patients treated with Peg-interferon (IFN)/ribavirin have achieved sustained clinical and virologic responses in up to 60% of cases.8 Although this approach affords a satisfactory response rate, additional therapy may be needed in MC patients with severe organ involvement and/or without an early virologic response.7,8
The efficacy of anti-CD20 monoclonal antibody (rituximab) has been reported in patients with HCV-MC vasculitis.5,6,10-16 A complete clinical response is achieved in 60% to 70% of cases, with cryoglobulin clearance in two-thirds of the patients.5,6,10,11,13-16 The absence of efficacy on HCV viral clearance and, furthermore, the potential increase in HCV viral load stress the need for combined antiviral therapy to block the HCV infection trigger. Indeed, up to 40% of patients treated with rituximab experience vasculitis relapse during B-cell recovery.5,6,10,11,13-16 However, no study has compared rituximab plus Peg-IFN-α/ribavirin to Peg-IFN-α/ribavirin in HCV-MC.
The goal of the present study was to determine the benefit of a treatment with rituximab plus Peg-IFN-α/ribavirin compared with Peg-IFN-α/ribavirin alone to better define the place of rituximab in the therapeutic strategy of HCV-MC. To this end, the clinical, virologic, and immunologic long-term outcome of 93 consecutive HCV-MC patients was analyzed. The efficacy of the combination of rituximab plus Peg-IFN-α/ribavirin (n = 38) was compared with Peg-IFN-α/ribavirin (n = 55).
Methods
Patients
Ninety-three consecutive, unselected patients with HCV-related MC vasculitis seen in the Department of Internal Medicine (Hôpital La Pitié-Salpétrière, Paris, France) between 2001 and 2008 were included. They had a serum cryoglobulin greater than 0.05 g/L on at least 2 occasions, which was associated with purpura, arthralgia,3 and sometimes with renal or neurologic involvement. All patients were positive for HCV RNA. Patients had histologically proven chronic active liver disease. Inclusion criteria for the study were as follows: (1) chronic active HCV infection, (2) signs of MC vasculitis, and (3) a minimum of 6 months of follow-up after discontinuing treatment (rituximab or antiviral therapy). Exclusion criteria were the presence of hepatitis B surface antigen or anti-HIV antibodies. Seventy patients had histologically confirmed systemic vasculitis (nerve, n = 41; kidney, n = 20; and skin, n = 9). Among those without histologically proven vasculitis (n = 23), patients with purpura were considered to have small-vessel vasculitis; and in the context of HCV infection and MC, they were classified as having MC vasculitis. The clinical evaluation included age, sex, recent weight loss, neurologic involvement (peripheral and/or central nervous system), cutaneous involvement (Raynaud phenomenon, purpura, distal ulcers), arthralgia, myalgia, sicca syndrome, gastrointestinal tract involvement, renal involvement (proteinuria, hematuria, and glomerular filtration rate), and clinical signs of hepatic involvement. The diagnosis of non-Hodgkin lymphoma was based on World Health Organization criteria.17
Study design
This was a prospective cohort study, including consecutive, unselected HCV-MC patients. All patients received antiviral therapy with Peg-IFN-α (2a, 180 μg/week, n = 5; or 2b, 1.5 μg/kg per week, n = 88, subcutaneously) plus ribavirin (600-1200 mg/day orally) for 48 weeks. For the 38 patients who received the combination of rituximab plus Peg-IFN-α/ribavirin, the therapeutic schedule consisted of: (1) weekly administration of 4 intravenous infusions of rituximab at 375 mg/m2 (on days 1, 8, 15, and 22; n = 31) or 2 intravenous infusions of rituximab at 1000 mg (on days 1 and 15; n = 7) followed 1 month later by the antiviral combination with Peg-IFN-α/ribavirin for 48 weeks. Preliminary results in 16 of them have been previously reported.18 The remaining 55 HCV-MC patients were treated by Peg-IFN-α/ribavirin alone. In accordance with our previous reports,7,8 treatment duration was 12 months for all genotypes with Peg-IFN-α and ribavirin. The assignment of therapy was decided by the clinician in charge of the patient. The study was approved by the institutional ethical committee of Pitié-Salpétrière Hospital, and written consent was obtained from all patients in accordance with the Declaration of Helsinki. None of the patients had received previous treatment with rituximab. Rituximab and antiviral therapy were not started at the same time to avoid cumulative side effects.18 Premedication with methylprednisolone 40 mg intravenously was given before each infusion of rituximab. Patients were evaluated using the same schedule in both groups of therapy.
Immunologic and virologic markers
Laboratory evaluation included a complete hemogram, serum chemistry profile, rheumatoid factor, IgM level, C4 fraction of complement, and cryoglobulin. Cryoglobulins were measured as previously described.19 Cryoglobulins were classified according to the method described by Brouet et al as either type II MC, which includes a monoclonal component, or type III MC, defined by the association of polyclonal immunoglobulins.20 Glomerular filtration rate was determined as previously described by Cockcroft and Gault.21 A 24-hour urine collection was also performed to quantify daily protein excretion.
HCV antibodies were detected by 2 specific third-generation immunoassays (Monolisa anti-HCV Plus, Sanofi Diagnostic Pasteur; Axsym HCV, Version 3.0, Abbott). Serum HCV RNA was measured by a reverse-transcription polymerase chain reaction assay (Amplicor HCV test; Roche Diagnostics) with a threshold of detection of 2.7 log copies/mL. HCV genotyping was performed using a second-generation line probe assay (Innogenetics). Liver biopsy specimens were evaluated according to the previously validated METAVIR scoring system.22
Flow cytometry
The concentration (cells/mm3) of CD19+ B lymphocytes was established from fresh blood samples with a 4-color flow cytometric analysis at initial evaluation and every 3 months until 21 months. Peripheral blood mononuclear cells were isolated from blood sample using Ficoll-Hypaque density gradient centrifugation. Peripheral blood mononuclear cells were washed twice in phosphate-buffered saline (PBS) containing 2% fetal calf serum and numerated before labeling. The flow cytometric analysis of lymphocyte subpopulations was performed using a panel of monoclonal antibodies conjugated to fluorescein isothiocyanate, phycoerythrin (PE), peridinin chlorophyll protein, PE–cyanine 5, or allophycocyanin to the following cell surface proteins: PE-labeled anti-CD19 and allophycocyanin-labeled anti-CD20 were from Beckman Coulter, and PE-labeled anti-CD27, PE-cyanine 5–labeled anti-CD38, and fluorescein isothiocyanate–labeled anti-IgD were from BD Biosciences. Irrelevant antibodies of respective isotypes were used as negative controls. Briefly, cells were incubated with the appropriate antibodies at 4°C for 20 minutes, washed in PBS containing 2% fetal calf serum, and then fixed in PBS containing 1% paraformaldehyde. Cells acquisition and analysis by flow cytometry was performed using a FACSCalibur (BD Biosciences). The monoclonal antibody G6 was provided by Dr R. Jefferis (University of Birmingham, Birmingham, United Kingdom). G6 reacts with an epitope of the VH1-69 VH gene product.23
Treatment efficacy
The response to treatment was analyzed by comparing clinical, immunologic, and virologic parameters at the initial evaluation, at 1 month, 3 months, 6 months, 9 months, at the end of therapy, 6 months after stopping antiviral therapy, and at the end of follow-up. Clinical response was defined by analyzing the progression of the following main clinical signs: skin involvement (absence of purpura and/or leg ulcer), peripheral neuropathy (clinical and electrophysiologic improvement on 2 successive examinations), renal involvement (normalization of serum creatinine level and disappearance of proteinuria and/or hematuria), and the absence of arthralgia. For patients with peripheral neuropathy, detailed clinical improvement or disappearance of neuropathic symptoms and muscle strength was evaluated according to the Medical Council of Great Britain serial, ranging from 0 (complete paralysis) to 5 (normal strength), and electrophysiologic parameters (ie, motor and sensory conduction) obtained at the time of the initial examination, during the follow-up, and after antiviral therapy were analyzed.
A complete clinical response of MC vasculitis was defined by an improvement in all baseline clinical manifestations. A partial response was defined by an improvement of at least half of the baseline clinical manifestations. All other patients were classified as nonresponders. The delay to achieve a complete response was analyzed using the same schedule in both groups of therapy, by systematic clinical evaluation at 1, 3, 6, 9, 12, and 18 months after starting therapy. A sustained virologic response was defined by the absence of detectable serum HCV RNA 6 months after stopping antiviral treatment; the remaining patients were classified as virologic nonresponders. A complete immunologic response was defined by the absence of serum cryoglobulin, and a partial immunologic response by a more than 50% decrease in the baseline cryoglobulin level. Clinical relapse was defined as the reappearance of clinical signs of vasculitis, virologic relapse as the reappearance of detectable HCV RNA, and immunologic relapse as the reappearance or increase of serum cryoglobulin 6 months after stopping antiviral therapy.
The time point to evaluate the overall response rate to therapy was at the end of 12 months of antiviral therapy. Relapses and sustained virologic response were evaluated 6 months after stopping antiviral therapy (ie, 18 months after starting antiviral therapy). Complete lymphoma remission was defined as the total disappearance of the lymphomatous mass; a partial remission was defined as a 75% reduction.
Statistical analysis
Data are expressed as the mean plus or minus SD or median (Q1;Q3). Categorial variables were compared using Fisher exact or χ2 tests, and continuous variables using the t test or Mann-Whitney U test when appropriate. Comparisons between baseline and end of follow-up values were tested using the MacNemar test or the Wilcoxon paired test. Overall survival was estimated using the Kaplan-Meier method. All tests were 2-sided at a .05 significance level. Analyses were performed using GraphPad Prism Version 4.0 (GraphPad Software).
Results
Characteristics of the HCV-MC patients
Patient characteristics are detailed in Table 1. Ninety-three HCV-MC patients, with a median age of 60 years (Q1 49; Q3 69) were included. The mean duration of HCV-MC vasculitis was 26.5 months. Main clinical features of MC included purpura (66.6%), polyneuropathy (72%), arthralgia (38.7%), kidney involvement (33.3%), and central nervous system involvement (8.6%). Thirteen patients (13.9%) had B-cell non-Hodgkin lymphoma (marginal zone lymphoma, n = 8; lymphoplasmacytic, n = 4; and lymphocytic, n = 1).
MC was present in 96.8%, with a median cryoglobulin level of 0.8 g/L (Q1 0.45; Q3 1.5). Seventy-eight patients (83.9%) had a type II cryoglobulin with monoclonal IgMk in all cases except 5 (IgGk in 3 and IgGλ in 2). C4 and CH50 serum levels were low in 87.5% of patients. HCV genotypes was type 1 in 67.7%. Sixty-seven patients (72%) had elevated serum alanine aminotransferase levels, and the mean alanine aminotransferase concentration was 2.2-fold the upper limit of normal value. The mean HCV RNA level was 5.8 plus or minus 0.7 log copies/mL. On liver biopsy, all patients had signs of chronic active hepatitis, with a mean METAVIR activity score of 1.3 plus or minus 0.8 and a mean fibrosis score of 2.1 plus or minus 1.3.
Forty-two patients (45.2%) were naive to antiviral therapy, whereas the 51 (54.8%) remaining were resistant to a previous antiviral therapy (Table 1).
Treatment efficacy
The main treatment-related data are summarized in Tables 2 and 3. Sixty-eight patients (73.1%) were complete clinical responders, 22 (23.6%) were partial responders, and 3 (3.2%) were nonresponders (Table 2). Clinical improvement was observed after a mean time of 6.8 plus or minus 4.7 months. The time of clinical remission was shorter in the rituximab plus Peg-IFN-α/ribavirin group (5.4 ± 4.0 months) compared with the Peg-IFN-α/ribavirin group (8.4 ± 4.7 months, P = .004). A similar rate of complete clinical responders was found in the 2 treatment group (72.7% in the Peg-IFN-α/ribavirin group vs 73.7% in the rituximab plus Peg-IFN-α/ribavirin group; Table 2).
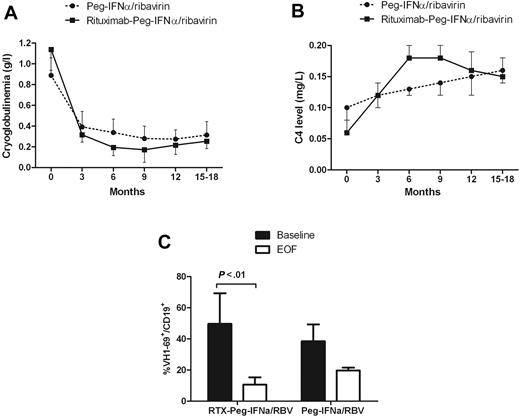
The immunologic response rate was higher in the rituximab plus Peg-IFN-α/ribavirin group (68.4% vs 43.6%, P = .001; Table 2). The cryoglobulin level decreased significantly more in the rituximab plus Peg-IFN-α/ribavirin group (1.1-0.17 g/L at month 9, P < .001) compared with Peg-IFN-α/ribavirin (0.8-0.28 g/L at month 9, P < .01; Figure 1A). The C4 serum level increased from 0.10 to 0.16 g/L in the Peg-IFN-α/ribavirin group and from 0.06 to 0.15 g/L under rituximab plus Peg-IFN-α/ribavirin (Figure 1B).
Course of immunologic parameters. Cryoglobulinemia (A), C4 serum level (B), and VH1-69+ B-cell clones (C) in HCV-MC patients according to the type of treatment. RTX indicates rituximab; RBV, ribavirin; and EOF, end of follow-up.
Course of immunologic parameters. Cryoglobulinemia (A), C4 serum level (B), and VH1-69+ B-cell clones (C) in HCV-MC patients according to the type of treatment. RTX indicates rituximab; RBV, ribavirin; and EOF, end of follow-up.
We next asked whether treatments alter Vh1-69 clonal B-cell production (Figure 1C). Vh1-69 heavy chain rearrangement was identified in high frequency among patients with HCV-induced MC vasculitis and lymphoproliferation.24 It is thought that Vh1-69-positive cells may represent B cells that are trying to mount an immunoglobulin response against the E2 viral envelope protein and commonly undergo clonal proliferation.24 We have studied Vh1-69 rearrangement with a specific antibody directed against the Vh1-69 gene product (G6). The proportion of Vh1-69 (G6+) positive cells among CD19+ populations at baseline was 49.7% plus or minus 10% in the rituximab plus Peg-IFN-α/ribavirin group and 38.5% plus or minus 19% in the Peg-IFN-α/ribavirin group. After therapy, the Vh1-69-positive cell proportion in HCV-MC patients decreased to 10.6% plus or minus 4% (P < .01) in the rituximab plus Peg-IFN-α/ribavirin and to 19.7% plus or minus 2% (P = .12) in the Peg-IFN-α/ribavirin.
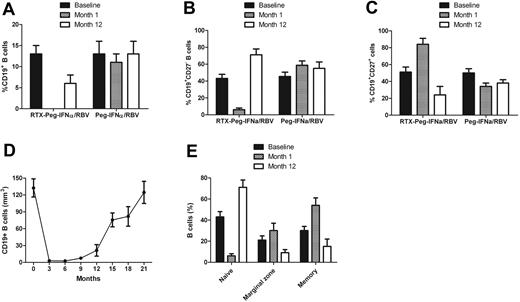
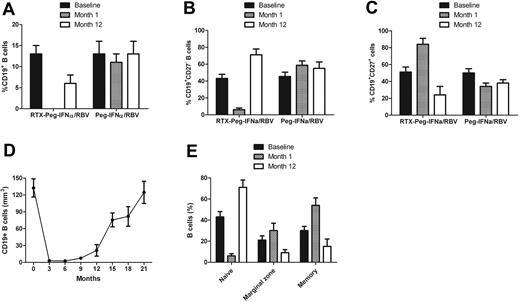
B-cell depletion in the peripheral blood was achieved in all patients except 1 in the rituximab plus Peg-IFN-α/ribavirin group (Figures 2–3). The mean percentage of CD19+ cells at baseline was 13.7% and dropped to 0% after the fourth infusion of rituximab. Detailed analysis of the course of B-cell subpopulations (naive, IgD+CD27−CD38low; memory, IgD−CD27+CD38low; marginal zone B cells, IgD+CD27+) under rituximab plus Peg-IFN-α/ribavirin therapy is represented in Figure 2E. Recovery of the B-cell count began after 9 months. There was a delayed B-cell reconstitution that actually started at the end of antiviral therapy (3.9% at month 12 and 8.7% at month 15). The mean percentage of CD19+CD27− B cells was 43% plus or minus 5% at baseline in the rituximab plus Peg-IFN-α/ribavirin group and dropped to 6% plus or minus 2% (P < .001) at month 1, whereas it was 45% plus or minus 5% at baseline and 59% plus or minus 5% at month 1 in the Peg-IFN-α/ribavirin group (Figure 2B). The mean percentage of CD19+CD27+ B cells was 51% plus or minus 6% at baseline in the rituximab plus Peg-IFN-α/ribavirin group and increased to 84% plus or minus 7% (P < .001) at month 1. In contrast, the mean percentage of CD19+CD27+ B cells was 50% plus or minus 5% at baseline and decreased to 34% plus or minus 4% (P < .05) at month 1 in the Peg-IFN-α/ribavirin group (Figure 2C).
Course of B-cell total population and subpopulations in HCV-MC patients according to the type of treatment. (A) CD19+ B cells. (B) Naive B cells. (C) Memory B cells. (D) B-cell depletion after rituximab plus Peg-IFN-α/ribavirin. (E) Analysis of B-cell subpopulations after rituximab plus Peg-IFN-α/ribavirin. RTX indicates rituximab; and RBV, ribavirin.
Course of B-cell total population and subpopulations in HCV-MC patients according to the type of treatment. (A) CD19+ B cells. (B) Naive B cells. (C) Memory B cells. (D) B-cell depletion after rituximab plus Peg-IFN-α/ribavirin. (E) Analysis of B-cell subpopulations after rituximab plus Peg-IFN-α/ribavirin. RTX indicates rituximab; and RBV, ribavirin.
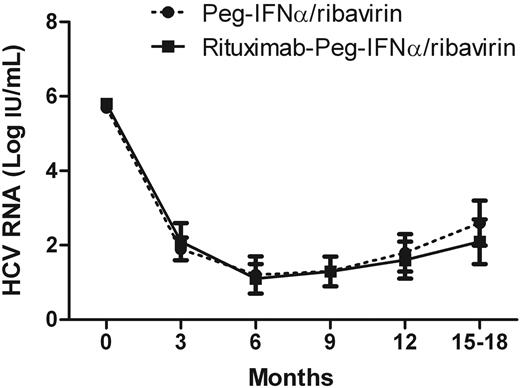
Course of HCV viral load in HCV-MC patients according to the type of treatment. RBV indicates ribavirin.
Course of HCV viral load in HCV-MC patients according to the type of treatment. RBV indicates ribavirin.
The virologic response did not differ between the 2 groups, with 60% and 57.9% of sustained virologic responders in the Peg-IFN-α/ribavirin and rituximab plus Peg-IFN-α/ribavirin group, respectively (Table 2). Antiviral therapy induced a significant decrease in the HCV viral load from 5.8 plus or minus 0.8 to 1.2 plus or minus 1.2 log copies/mL in the Peg-IFN-α/ribavirin group and from 5.8 plus or minus 0.6 to 1.1 plus or minus 0.9 log copies/mL in the rituximab plus Peg-IFN-α/ribavirin group (Figure 3). The alanine aminotransferase level did not increase in any patients. Alanine aminotransferase levels decreased from 2.2 to 1.3 the upper limit of normal value and normalized in 56 of 93 (60.2%) patients.
Multivariate analysis was performed to assess factors influencing virologic response. Severe liver fibrosis (METAVIR F3-F4) was independently associated with viral persistence (odds ratio = 0.2; 95% confidence interval, 0.06-0.68, P = .008). Although not significant, a trend toward an association with viral persistence and HCV genotype 1 (odds ratio = 0.45; 95% confidence interval, 0.13-1.54, P = .20) was observed.
Purpura improved in 78 (83.8%), arthralgia in 75 (80.6%), kidney involvement in 21 (67.7%), and polyneuropathy in 34 (50.7%) patients. No significant difference was found in the clinical response rate between the 2 treatment regimens according to main organs involved, except for kidney involvement (Table 3). There was a higher rate of complete remission of kidney involvement in patients treated with rituximab plus Peg-IFN-α/ribavirin (17 of 21, 80.9%) compared with those receiving Peg-IFN-α/ribavirin (4 of 10, 40%, P = .04). Detailed analysis of the kidney response is summarized in Table 3.
Complete response of B-cell non-Hodgkin lymphoma was achieved in 4 of 6 patients (66.6%) in the Peg-IFN-α/ribavirin group and 7 of 7 (100%) in the rituximab plus Peg-IFN-α/ribavirin group.
Long-term follow-up
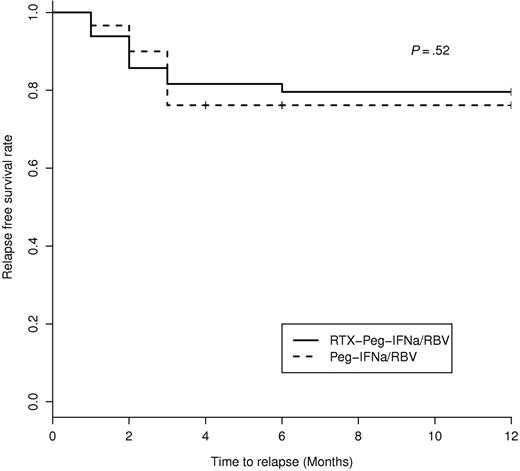
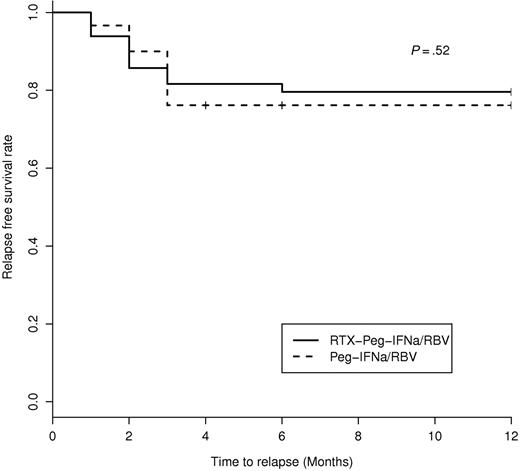
Seventeen patients (18.3%) experienced a clinical relapse, including 10 patients treated with Peg-IFN-α/ribavirin and 7 with rituximab plus Peg-IFN-α/ribavirin. Relapses occurred 3 plus or minus 1 months after discontinuing antiviral therapy and 15 plus or minus 7 months after the initiation of rituximab (in the rituximab plus Peg-IFN-α/ribavirin group; Figure 4). All clinical relapses were associated with an immunologic relapse. All clinical and immunologic relapsers (n = 17) were also virologic relapsers, except in 6 patients, of whom 1 had B-cell lymphoma and the 5 remaining had relapse of MC despite negative HCV RNA as previously described,25 in the Peg-IFN-α/ribavirin group. Among the 10 clinical relapsers in the Peg-IFN-α/ribavirin group, retreatment included rituximab (n = 2), rituximab plus Peg-IFN-α/ribavirin (n = 1), and Peg-IFN-α/ribavirin (n = 7) associated with a low dose of prednisone in 4 cases. After retreatment, 8 of 10 patients were clinical complete responders and the 2 remaining were partial responders. Among the 7 clinical relapsers in the rituximab plus Peg-IFN-α/ribavirin group, 5 of 7 (71.4%) experienced a B-cell recovery between months 12 and 15, and 1 of 7 did not experience B-cell recovery. There was no B-cell depletion in the remaining relapser. In the rituximab plus Peg-IFN-α/ribavirin group, retreatment of clinical relapsers included rituximab (n = 2), rituximab plus cyclophosphamide (n = 1), and Peg-IFN-α/ribavirin.(4) After retreatment, 5 of 7 were clinical complete responders and the 2 remaining were partial responders.
Kaplan-Meier estimation of relapse-free survival in HCV-MC patients according to the type of treatment.
Kaplan-Meier estimation of relapse-free survival in HCV-MC patients according to the type of treatment.
The overall median follow-up was 48 months (Q1 24 months; Q3 60 months). It was 24 months (Q1 12 months; Q3 36 months) in the rituximab plus Peg-IFN-α/ribavirin group versus 60 months (Q1 48 months; Q3 72 months) in the Peg-IFN-α/ribavirin arm. Five patients (5.4%) died of liver carcinoma (n = 3), cirrhosis (n = 1), or unknown cause (n = 1). The median time to death after therapy was 36 months (Q1 8 months; Q3 44 months). No difference was noted in the 2 groups of therapy according to death. At the end of follow-up, overall complete clinical, immunologic, and virologic responses were achieved in 68.8% (64 of 93), 43% (40 of 93), and 59.1% (55 of 93) HCV-MC patients, respectively.
Tolerance of treatment
Table 4 summarizes the tolerance profile of the 2 regimens. Antiviral therapy and rituximab were well tolerated in 45.2% and 73.7%, respectively. Main side effects of antiviral therapy included fatigue (47.3%), fever and anemia (37.6%), leukoneutropenia (20.4%), thrombocytopenia (18.3%), depression (13.9%), and pruritus (6.4%). Antiviral therapy interruption was required in 10 patients (10.7%) because of hematologic toxicity (n = 5), depression (n = 2), flare-up of skin psoriasis (n = 1), neuropathy (n = 1), and hepatocarcinoma (n = 1). Side effects of rituximab included serum sickness (10.5%), neutropenia and Streptococcus pneumoniae pneumopathy (5.2%), varicella-zoster virus infection, and erysipela (n = 1).
Discussion
In the present study, we compared the efficacy and tolerance of rituximab plus Peg-IFN-α/ribavirin with Peg-IFN-α/ribavirin in patients with HCV-MC vasculitis. Our aim was to determine the benefit of using antiviral therapy with or without rituximab in HCV-MC to better define the place of rituximab in the therapeutic strategy of HCV-MC. The most striking conclusions drawn by this study are: (1) the higher efficacy of rituximab plus Peg-IFN-α/ribavirin on kidney involvement (ie, membrano-proliferative glomerulonephritis), (2) the shorter time to achieve a complete clinical response, (3) the higher immunologic efficacy, and (4) the synergistic effect of rituximab and antiviral therapy on clonal B cells compared with Peg-IFN-α/ribavirin therapy alone.
The 2 therapeutic groups were comparable in terms of age, virologic and immunologic parameters, and previous antiviral therapy (ie, 45% were naive of antiviral therapy). The main clinical features of vasculitis were comparable, except for the higher frequency of kidney involvement in the rituximab plus Peg-IFN-α/ribavirin group. Nephropathy is considered as a major cause of morbidity and mortality in MC.26,27 The use of rituximab in association with antiviral therapy increased by 2 times the odds of achieving a remission of kidney lesions. Indeed, 81% of HCV-MC patients treated with rituximab plus Peg-IFN-α/ribavirin had a complete remission of the membrano-proliferative glomerulonephritis compared with 40% in the Peg-IFN-α/ribavirin group. Despite severe renal impairment (mean baseline glomerular filtration rate of 42.8 mL/min), all the kidney parameters significantly improved after rituximab plus Peg-IFN-α/ribavirin. In contrast, patients treated by Peg-IFN-α/ribavirin did not experience significant change in serum creatinine level or glomerular filtration rate. These results are reminiscent of our previous report showing that HCV-MC patients with a glomerular filtration rate lower than 70 mL/minute were 5.6 times less likely to be complete clinical responders to antiviral therapy.8 Renal manifestations of MC are probably the result of immune complexes or cryoglobulin deposits and decreased clearance of IC.28-30 Vascular skin and glomerular lesions associated with cryoglobulinemia can be induced in normal mice by the injection of a monoclonal antibody exhibiting both cryoglobulin and rheumatoid factor activities derived from the MRL-lpr/lpr autoimmune mouse.29
Rituximab shortens the delay of achieving a complete clinical reponse. The mean time for complete clinical response was 5 months with rituximab plus Peg-IFN-α/ribavirin group. This was 3 months earlier than with Peg-IFN-α/ribavirin therapy alone. Our results are consistent with those reported after rituximab use as a single agent showing a complete clinical response of HCV-MC 5 to 6 months after infusions.5 These findings are probably clinically meaningful because corticosteroids and cytotoxic agents are associated with considerable morbidity and do not improve HCV-MC vasculitic manifestations.26,31,32 Several groups have reported on the efficacy of rituximab, as a single agent, in HCV-MC.5,10,11,13-16,33 Rituximab infusions proved effective on main vasculitis signs, with a complete clinical response in 73% of patients for skin involvement, 53% for arthralgia, 36% for neuropathy, and 70% for glomerulonephritis. However, cryoglobulinemic vasculitis relapse was noted in 36.1% of patients within a few days to 19 months after the last rituximab infusion.5,10,11,13-16,33
Rituximab combined with Peg-IFN-α/ribavirin exerted a synergistic effect on clonal B-cell expansions. VH1-69 clonal B cells dramatically decreased after rituximab and antiviral therapy as previously reported with rituximab as a single agent.34 Clonal expansion of marginal zone–like IgM+ CD27+ B cells has been recently observed in certain HCV-MC patients.35 The finding that these memory-like B cells expressed somatically hypermutated immunoglobulins suggests specific antigenic stimulation.35 Interestingly, we previously reported that VH1-69 clonal B cells were mostly marginal zone–like B cells in MC.34 In the present study, rituximab plus Peg-IFN-α/ribavirin was more efficient to suppress both memory and VH1-69 clonal B cells compared with Peg-IFN-α/ribavirin alone. Complete immunologic response was higher with the combination of rituximab plus Peg-IFN-α/ribavirin. Cryoglobulin clearance is a major goal of MC therapy because recurrence of vasculitis, sometimes associated with B-cell lymphoma, has been reported in patients with persistent cryoglobulin despite successful treatment of HCV infection.25,36 Indeed, in the Peg-IFN-α/ribavirin group, one-third of the vasculitis relapses occurred despite a sustained virologic response with overt B-cell lymphoma in one patient. B-cell proliferation may eventually reach an autonomous phase in which it may become HCV independent.37 Rituximab combined with Peg-IFN-α/ribavirin may delete both virus-dependent and -independent B-cell clones. Antiviral therapy alone decreased the memory B cells; whereas in association with rituximab, naive B cells are the main depleted population. These results may account for the delayed B-cell reconstitution after rituximab plus Peg-IFN-α/ribavirin18 and stresses the synergistic action of rituximab and antiviral therapy at the immunologic level.
Relapse rate concerned less than 20% of HCV-MC patients after a median follow-up of 48 months. This was significantly lower than the 40% reported with rituximab as a single agent.5,6 In this setting, rituximab cannot be seen as a curative treatment as long as the viral starter antigen of the vasculitis (ie, HCV) remains. Multivariate analysis showed that HCV-MC patients with severe liver fibrosis were 5 times less likely sustained virologic responders. The safety profile of rituximab combined with Peg-IFN-α/ribavirin was satisfactory with only mild to moderate side effects. No rise of HCV viral load was observed under rituximab. Treatment interruptions occurred in 11% of patients and were equally distributed between the 2 groups of therapy. All discontinuations were related to Peg-IFN-α/ribavirin therapy. However, caution is still warranted because we recently reported a severe early flare-up of MC vasculitis and serum sickness–like syndrome under rituximab, especially in patients with high level of serum cryoglobulin and with the use of 1-g infusions at days 1 and 15.38
In conclusion, rituximab combined with Peg-IFN-α/ribavirin is well tolerated and more effective than Peg-IFN-α/ribavirin in HCV-MC. Rituximab synergizes the immunologic effect of antiviral therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D. Saadoun and P.C. designed research; D. Saadoun, D. Sene, B.T., L.M., M.R., and P.C. performed research; D. Saadoun, D. Sene, B.T., A.K., Y.S., L.P., B.C., F.B., J.-C.P., and P.C. collected data; D. Saadoun and P.C. analyzed and interpreted data; D. Saadoun and M.R.R. performed statistical analysis; and D. Saadoun and P.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrice Cacoub, AP-HP, Hôpital Pitié-Salpêtrière, Service de Médecine Interne, 83 blvd de l'hopital, Paris, F-75013 France; e-mail: patrice.cacoub@psl.aphp.fr.