Abstract
In inflamed venules, neutrophils rolling on E-selectin induce integrin αLβ2-dependent slow rolling on intercellular adhesion molecule-1 by activating Src family kinases (SFKs), DAP12 and Fc receptor-γ (FcRγ), spleen tyrosine kinase (Syk), and p38. E-selectin signaling cooperates with chemokine signaling to recruit neutrophils into tissues. Previous studies identified P-selectin glycoprotein ligand-1 (PSGL-1) as the essential E-selectin ligand and Fgr as the only SFK that initiate signaling to slow rolling. In contrast, we found that E-selectin engagement of PSGL-1 or CD44 triggered slow rolling through a common, lipid raft–dependent pathway that used the SFKs Hck and Lyn as well as Fgr. We identified the Tec kinase Bruton tyrosine kinase as a key signaling intermediate between Syk and p38. E-selectin engagement of PSGL-1 was dependent on its cytoplasmic domain to activate SFKs and slow rolling. Although recruiting phosphoinositide-3-kinase to the PSGL-1 cytoplasmic domain was reported to activate integrins, E-selectin–mediated slow rolling did not require phosphoinositide-3-kinase. Studies in mice confirmed the physiologic significance of these events for neutrophil slow rolling and recruitment during inflammation. Thus, E-selectin triggers common signals through distinct neutrophil glycoproteins to induce αLβ2-dependent slow rolling.
Introduction
Circulating leukocytes enter inflamed tissues through sequential adhesive and signaling events.1 Neutrophils first tether to and roll on P- and E-selectin expressed on activated endothelial cells.2 They roll on P-selectin through interactions with P-selectin glycoprotein ligand-1 (PSGL-1)3,4 and on E-selectin through interactions with PSGL-1, CD44, and E-selectin ligand-1.5-7 Rolling neutrophils encounter immobilized chemokines that signal through Gαi protein–coupled receptors. These signals activate integrins αLβ2 and αMβ2 to their high-affinity states, enabling interactions with intercellular adhesion molecule-1 (ICAM-1) that promote arrest, adhesion strengthening, intraluminal crawling, and transendothelial migration.1
Importantly, E-selectin directly triggers signals in rolling neutrophils that cooperate with chemokine signals to maximize neutrophil recruitment during inflammation.8 In autoperfused whole blood, neutrophils rolling on immobilized E-selectin activate αLβ2 to an intermediate-affinity state, which slows rolling on coimmobilized ICAM-1.8 In vivo, neutrophils roll slowly on E-selectin expressed in tumor necrosis factor-α (TNF-α)–stimulated postcapillary venules.9 Injecting anti-αLβ2 antibody increases rolling velocities, documenting the physiologic importance of integrins for slow rolling in vivo.8,10 Integrin-dependent slow rolling prolongs transit times in inflamed venules11 and enhances neutrophil recruitment.8
In autoperfused whole blood, it was reported that integrin-mediated slow rolling on E-selectin and ICAM-1 is eliminated in neutrophils lacking PSGL-1 but only marginally impaired in neutrophils lacking CD44.8 This result suggests that PSGL-1 has unique signaling functions that are essential to activate β2-integrins. In vivo, however, wild-type (WT) and PSGL-1−/− neutrophils roll with similar velocities in TNF-α–stimulated venules,5,6 implying that at least one more E-selectin ligand induces integrin-mediated slow rolling.
E-selectin signaling exhibits similarities to immunoreceptor or integrin outside-in signaling. Engagement of immunoreceptors or integrins activates Src family kinases (SFKs).12-14 The SFKs phosphorylate tyrosines within immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic domains of adaptor proteins. After docking to the ITAMs, spleen tyrosine kinase (Syk) is also tyrosine phosphorylated by SFKs. Activated Syk propagates downstream activation of Tec kinases, phosphoinositide-3-kinase (PI3K), and p38 mitogen-activated protein kinase. Similarly, neutrophils rolling on E-selectin activate SFKs, ITAM adaptors, Syk, and p38.8,15-17 Other than p38, the signaling components downstream of Syk that induce integrin-mediated slow rolling have not been identified.
Neutrophils have 2 ITAM-bearing adaptors associated with immunoreceptors: DAP12 and Fc receptor-γ (FcRγ). Both are required for E-selectin to cause slow rolling on ICAM-1.15 Neutrophils have 3 SFKs: Fgr, Hck, and Lyn. Unexpectedly, genetic deletion of Fgr but not of Hck or Lyn was shown to prevent E-selectin–mediated Syk activation in neutrophils and slow rolling on ICAM-1.15 This result suggests a unique function for Fgr in E-selectin–triggered signaling. However, neutrophils lackingboth Hck and Lyn were not studied, and thus their roles in E-selectin–induced signaling were not excluded.
Fgr, Hck, and Lyn associate with cholesterol-dependent membrane rafts,18 and PSGL-1 and CD44 partition into rafts.19-21 It is not known whether E-selectin–triggered signaling requires lipid rafts, as observed for immunoreceptor signaling,13,14 or is independent of rafts, as reported for integrin outside-in signaling.22
In vitro, neutrophils rolling on P-selectin also trigger Syk-dependent activation of αLβ2 to slow rolling on coimmobilized ICAM-1.8,19 Neutrophils from knock-in mice that express PSGL-1 without the cytoplasmic domain roll normally on P-selectin but do not roll slower on ICAM-1.19 These data reveal a requirement for the cytoplasmic domain of PSGL-1 to initiate Syk-dependent integrin activation as neutrophils roll on P-selectin. Prolonged P-selectin binding to neutrophils activates integrin αMβ2 by recruiting PI3Kδ to the PSGL-1 cytoplasmic domain.23 Whether PSGL-1 requires its cytoplasmic domain or uses PI3K to trigger E-selectin–dependent slow rolling is not known.
Here we show, in contrast to earlier reports,8,15 that E-selectin engagement of either PSGL-1 or CD44 triggers integrin-mediated slow neutrophil rolling through a common pathway that uses all 3 SFKs to activate Syk. Furthermore, we identify the Tec kinase Bruton tyrosine kinase (Btk) as a key signaling intermediate between Syk and p38. Signaling requires intact lipid rafts. PSGL-1 needs its cytoplasmic domain to signal slow rolling on E-selectin as well as P-selectin, but PI3K is not required for slow rolling on either selectin. Studies in mice confirm the physiologic relevance of the signaling events. These results provide new mechanistic insights into the earliest signals received by neutrophils as they interact with the vessel wall under flow.
Methods
Reagents and mice
Cells, recombinant proteins, antibodies, mice, and the protocol for making radiation chimeras by bone marrow transplantation are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All studies with mice described in this paper were approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Flow chamber assay
Intravital microscopy
Thioglycollate-induced peritonitis
Details are provided in supplemental Methods.
Competitive neutrophil recruitment assay
Details are provided in supplemental Methods.
SFK, Syk, Btk, and p38 phosphorylation
Details are provided in supplemental Methods.
Results
Neutrophils use Hck and Lyn as well as Fgr to signal slow rolling on E-selectin or P-selectin
In our experimental system, isolated murine bone marrow leukocytes exhibit αLβ2- and Syk-dependent slow rolling on P-selectin and ICAM-1, with neutrophils accounting for more than 90% of the rolling cells.19 To further characterize the system, we perfused bone marrow leukocytes over E- or P-selectin with or without coimmobilized ICAM-1. WT neutrophils rolled more slowly on E- or P-selectin and ICAM-1 than on E- or P-selectin alone. Slow rolling was blocked by monoclonal antibodies (mAbs) to ICAM-1 or αLβ2 but not to αMβ2 (supplemental Figure 1A),19 by an inhibitor of SFKs (supplemental Figures 1B and 2A), in neutrophils lacking DAP12 and FcRγ (supplemental Figures 1C and 2B), and by inhibitors of Syk or p38 (supplemental Figures 1D and 2C). These results establish common signaling requirements for slow rolling of murine neutrophils from bone marrow or autoperfused whole blood.8,15 Furthermore, human neutrophils rolled more slowly on E- or P-selectin and ICAM-1 than on E- or P-selectin alone. Slow rolling was blocked by mAbs to ICAM-1 or αLβ2 but not to αMβ2 (supplemental Figures 4A and 5A)19 and by inhibitors of SFKs, Syk, or p38 (supplemental Figures 4C and 5C). Thus, human and murine neutrophils rolling on P- or E-selectin use similar kinases to induce αLβ2-dependent slow rolling on ICAM-1.
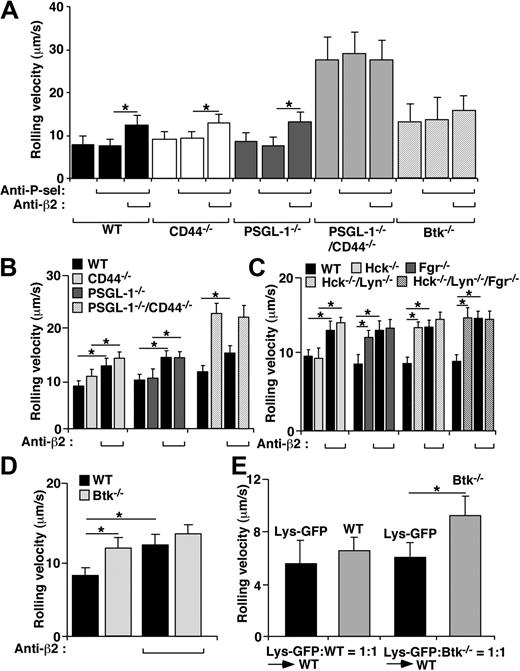
Previous studies showed that slow rolling on E-selectin and ICAM-1 is eliminated in Fgr−/− or Fgr−/−Hck−/−Lyn−/− neutrophils but not in Hck−/− or Lyn−/− neutrophils, suggesting that Fgr has a unique and nonredundant role in this pathway.15 We obtained similar results with neutrophils from these genotypes but also tested Hck−/−/Lyn−/− neutrophils. Expectedly, Hck−/−/Lyn−/− neutrophils had the same slow rolling defect on E- or P-selectin and ICAM-1 as Fgr−/− neutrophils (Figure 1A and supplemental Figure 3A). These findings demonstrate that Fgr cannot substitute for a function conferred by a combination of Hck and Lyn and suggest that each SFK, after activation by selectin engagement, phosphorylates a unique substrate(s).
Slow rolling of neutrophils on E-selectin and ICAM-1 requires Hck and Lyn as well as Fgr, lipid rafts, and Btk, but not PI3K. (A) Velocities of WT or SFK-deficient neutrophils rolling on E-selectin with or without coimmobilized ICAM-1. (B) Velocities of WT neutrophils rolling on E-selectin with or without coimmobilized ICAM-1 in the presence or absence of the vehicle control DMSO, MβCD or its inactive analog α-cyclodextrin (αCD), MβCD plus 15% FBS, or filipin III. (C) Velocities of WT neutrophils rolling on E-selectin with or without coimmobilized ICAM-1 in the presence or absence of the vehicle control DMSO, the PI3K inhibitor LY294002, or the Tec kinase inhibitor LFM-A13. (D) Velocities of WT, Btk−/−, SHIP1−/−, PI3Kγ−/−, or PI3Kδ−/− neutrophils rolling on E-selectin with or without coimmobilized ICAM-1. (E) Numbers of WT, SHIP1−/−, PI3Kγ−/−, or PI3Kδ−/− neutrophils rolling or firmly adherent (arrest) on coimmobilized E-selectin, ICAM-1, and CXCL1 in the presence or absence of the PI3K inhibitor LY294002. In all experiments, the E-selectin density was 200 sites/μm2 and the ICAM-1 density was 240 sites/μm2. The wall shear stress was 1 dyne/cm2. The data represent the mean ± SEM from 5 experiments. *P < .01.
Slow rolling of neutrophils on E-selectin and ICAM-1 requires Hck and Lyn as well as Fgr, lipid rafts, and Btk, but not PI3K. (A) Velocities of WT or SFK-deficient neutrophils rolling on E-selectin with or without coimmobilized ICAM-1. (B) Velocities of WT neutrophils rolling on E-selectin with or without coimmobilized ICAM-1 in the presence or absence of the vehicle control DMSO, MβCD or its inactive analog α-cyclodextrin (αCD), MβCD plus 15% FBS, or filipin III. (C) Velocities of WT neutrophils rolling on E-selectin with or without coimmobilized ICAM-1 in the presence or absence of the vehicle control DMSO, the PI3K inhibitor LY294002, or the Tec kinase inhibitor LFM-A13. (D) Velocities of WT, Btk−/−, SHIP1−/−, PI3Kγ−/−, or PI3Kδ−/− neutrophils rolling on E-selectin with or without coimmobilized ICAM-1. (E) Numbers of WT, SHIP1−/−, PI3Kγ−/−, or PI3Kδ−/− neutrophils rolling or firmly adherent (arrest) on coimmobilized E-selectin, ICAM-1, and CXCL1 in the presence or absence of the PI3K inhibitor LY294002. In all experiments, the E-selectin density was 200 sites/μm2 and the ICAM-1 density was 240 sites/μm2. The wall shear stress was 1 dyne/cm2. The data represent the mean ± SEM from 5 experiments. *P < .01.
Neutrophils require intact lipid rafts to signal slow rolling on E-selectin or P-selectin
We disrupted lipid rafts in murine neutrophils with methyl-β-cyclodextrin (MβCD) and filipin III, which act through different mechanisms.24 Neither MβCD nor filipin III altered the velocities of neutrophils rolling on E-selectin (Figure 1B) or P-selectin (supplemental Figure 3B) or the number of neutrophils rolling on either selectin (data not shown). However, both agents prevented integrin-dependent slow rolling on coimmobilized ICAM-1 (Figure 1B and supplemental Figure 3B) without affecting the number of rolling cells. Addition of serum to restore membrane cholesterol blocked the effects of MβCD, and addition of vehicle alone or of the inactive analog α-cyclodextrin did not prevent slow rolling (Figure 1B and supplemental Figure 3B). Identical results were observed with human neutrophils (supplemental Figures 4B and 5B).
Neutrophils require the Tec kinase Btk to signal slow rolling on E-selectin or P-selectin
Syk signals in part by activating Tec family kinases.25 Neutrophils express the Tec family kinases Btk, Tec, and Bmx.26 Btk−/− neutrophils or WT neutrophils treated with the Tec kinase inhibitor LFM-A1327 rolled with normal velocities on E-selectin (Figure 1C-D) or P-selectin (supplemental Figure 3C-D) but failed to slow rolling on coimmobilized ICAM-1. Thus, Btk is a key signaling intermediate for slow rolling. The Tec kinase inhibitor LFM-A13 also blocked slow rolling of human neutrophils on ICAM-1 immobilized with E- or P-selectin (supplemental Figures 4C and 5C).
Neutrophils do not require PI3K to signal slow rolling on E-selectin or P-selectin
During immunoreceptor signaling, Btk may act downstream of PI3K.28 Prolonged P-selectin binding to neutrophils recruits PI3Kδ to the PSGL-1 cytoplasmic domain to activate integrin αMβ2.23 However, the PI3K inhibitor LY294002 did not affect the velocities of murine neutrophils rolling on E-selectin or P-selectin and did not inhibit slow rolling on coimmobilized ICAM-1 (Figure 1C and supplemental Figure 3C). Identical results were observed with human neutrophils (supplemental Figures 4C and 5C). Furthermore, neutrophils from mice lacking PI3Kγ or PI3Kδ rolled normally on each selectin and rolled slower on ICAM-1 (Figure 1D and supplemental Figure 3D). We conducted a reciprocal experiment with mice lacking SH2-containing phosphatidylinositol-3,4,5-triphosphate 5-phosphatase 1 (SHIP1). SHIP1−/− leukocytes express high levels of the PI3K product phosphatidylinositol-3,4,5-triphosphate (PIP3) due to defective conversion of PIP3 to phosphatidylinositol-3,4-bisphosphate.29 Nevertheless, SHIP1−/− and WT neutrophils rolled similarly on E-selectin or P-selectin and exhibited similar slow rolling on coimmobilized ICAM-1 (Figure 1D and supplemental Figure 3D). As a positive control, we confirmed the role of PIP3 for integrin-dependent adhesion strengthening in chemokine-stimulated neutrophils.30 The chemokine CXCL1 signals through the Gαi protein–coupled receptor CXCR2 on neutrophils, causing the cells to arrest and spread in inflamed venules.31 WT neutrophils rolling on E-selectin (Figure 1E) or P-selectin (supplemental Figure 3E) coimmobilized with CXCL1 and ICAM-1 rapidly arrested and spread. PI3Kγ−/− and PI3Kδ−/− neutrophils had partial defects in arrest and spreading. The PI3K inhibitor LY294002 inhibited conversion from rolling to arrest. SHIP1-deficient neutrophils arrested and spread even more rapidly, which was inhibited by LY294002 (Figure 1E and supplemental Figure 3E). These data demonstrate that neither E-selectin– nor P-selectin–mediated slow rolling requires PI3K.
E-selectin engages PSGL-1 and CD44 through a common signaling pathway to slow neutrophil rolling
Because CD44 has other signaling capabilities,6,7 we reassessed its ability to induce integrin-dependent slow rolling. WT, PSGL-1−/−, and CD44−/− bone marrow neutrophils rolled with indistinguishable velocities on E-selectin alone, whereas PSGL-1−/−/CD44−/− neutrophils rolled significantly faster (Figure 2A). Unexpectedly, WT, PSGL-1−/−, and CD44−/− neutrophils exhibited comparable slow rolling on ICAM-1, whereas PSGL-1−/−/CD44−/− neutrophils did not roll slower on ICAM-1. We doubled the E-selectin density (400 sites/μm2) to increase the number of E-selectin/ligand bonds per unit time, which matched the rolling velocity of PSGL-1−/−/CD44−/− neutrophils with that of WT neutrophils rolling on E-selectin at 200 sites/μm2. PSGL-1−/−/CD44−/− neutrophils still failed to slow rolling on ICAM-1 (Figure 2A). As with WT neutrophils, slow rolling of PSGL-1−/− or CD44−/− neutrophils was eliminated by disrupting lipid rafts (Figure 2B-C) and by inhibitors of SFKs, Syk, Tec kinases, and p38, but not of PI3K (Figure 2D-E). These data demonstrate that E-selectin engagement of PSGL-1 or CD44 triggers a common signaling pathway to slow rolling on ICAM-1.
E-selectin engages PSGL-1 and CD44 through a common signaling pathway to slow neutrophil rolling. (A) Velocities of neutrophils from the indicated genotype rolling on E-selectin at the indicated site density with or without coimmobilized ICAM-1. (B) CD44−/− neutrophils or (C) PSGL-1−/− neutrophils rolling on E-selectin with or without coimmobilized ICAM-1 in the presence or absence of vehicle control DMSO, α-cyclodextrin (αCD), MβCD, or MβCD with serum. (D) CD44−/− neutrophils or (E) PSGL-1−/− neutrophils rolling on E-selectin with or without coimmobilized ICAM-1 in the presence or absence of DMSO, the Syk inhibitor piceatannol, the SFK inhibitor PP2 or its inactive analog PP3, the PI3K inhibitor LY294002, the Tec kinase inhibitor LFM-A13, or the p38 inhibitor SB203580. Except where indicated in panel A, the E-selectin density was 200 sites/μm2. The ICAM-1 density was 240 sites/μm2. The wall shear stress was 1 dyne/cm2. The data represent the mean ± SEM from 5 experiments. *P < .01.
E-selectin engages PSGL-1 and CD44 through a common signaling pathway to slow neutrophil rolling. (A) Velocities of neutrophils from the indicated genotype rolling on E-selectin at the indicated site density with or without coimmobilized ICAM-1. (B) CD44−/− neutrophils or (C) PSGL-1−/− neutrophils rolling on E-selectin with or without coimmobilized ICAM-1 in the presence or absence of vehicle control DMSO, α-cyclodextrin (αCD), MβCD, or MβCD with serum. (D) CD44−/− neutrophils or (E) PSGL-1−/− neutrophils rolling on E-selectin with or without coimmobilized ICAM-1 in the presence or absence of DMSO, the Syk inhibitor piceatannol, the SFK inhibitor PP2 or its inactive analog PP3, the PI3K inhibitor LY294002, the Tec kinase inhibitor LFM-A13, or the p38 inhibitor SB203580. Except where indicated in panel A, the E-selectin density was 200 sites/μm2. The ICAM-1 density was 240 sites/μm2. The wall shear stress was 1 dyne/cm2. The data represent the mean ± SEM from 5 experiments. *P < .01.
E-selectin engagement of PSGL-1 or CD44 activates SFKs and Syk in a lipid raft–dependent manner
We rotated murine leukocytes on immobilized E-selectin for 5 minutes, lysed the cells, and immunoprecipitated Fgr, Hck, or Lyn. Activation of each SFK was monitored by Western blotting with an antibody that recognizes phosphotyrosine 416 in the catalytic domain. As previously reported,15 E-selectin activated both Fgr and Hck, but unlike the previous report, E-selectin also activated Lyn (Figure 3A). Furthermore, P-selectin activated all 3 SFKs (supplemental Figure 6). E-selectin activated SFKs in WT, PSGL-1−/−, or CD44−/− neutrophils (Figure 3B). Even at higher densities, E-selectin did not activate SFKs in PSGL-1−/−/CD44−/− neutrophils (Figure 3B). Disrupting lipid rafts with MβCD prevented activation of SFKs (Figure 3C).
E-selectin engagement of PSGL-1 or CD44 activates SFKs in a lipid raft–dependent manner and then sequentially activates Syk, Btk, and p38. (A) WT leukocytes were rotated on E-selectin–IgM in the presence or absence of EDTA (ethylenediaminetetraacetic acid) or on control CD45-IgM for 5 minutes. Lysates were immunoprecipitated (IP) with antibody to the indicated SFK and then Western blotted (WB) with the same antibody or with anti–phospho-SFK (Y416). (B) Leukocytes of the indicated genotype were incubated as in panel A. Lysates were probed with antibody that recognizes all SFKs or with anti–phospho-SFK (Y416). (C) WT leukocytes treated with the indicated agent were incubated as in panel A. Lysates were probed with antibody that recognizes all SFKs or with anti–phospho-SFK (Y416). (D) WT leukocytes treated with the indicated agent or leukocytes of the indicated genotype were incubated as in panel A. Lysates were probed with antibody that recognizes all SFKs or with anti–phospho-SFK (Y416). (E) WT leukocytes treated with the indicated agent were incubated as in panel A. Lysates were probed with antibody to Syk or to phospho-Syk. (F) Lysates were Western blotted with antibody to Syk or to phospho-Syk. (G) Lysates were immunoprecipitated with antibody to Btk and then Western blotted with the same antibody or with antiphosphotyrosine antibody. (H) Lysates were probed with antibody to p38 or to phospho-p38. The E-selectin density was 400 sites/μm2 for PSGL-1−/−/CD44−/− leukocytes and 200 sites/μm2 for leukocytes of all other genotypes. The data are representative of at least 3 independent experiments.
E-selectin engagement of PSGL-1 or CD44 activates SFKs in a lipid raft–dependent manner and then sequentially activates Syk, Btk, and p38. (A) WT leukocytes were rotated on E-selectin–IgM in the presence or absence of EDTA (ethylenediaminetetraacetic acid) or on control CD45-IgM for 5 minutes. Lysates were immunoprecipitated (IP) with antibody to the indicated SFK and then Western blotted (WB) with the same antibody or with anti–phospho-SFK (Y416). (B) Leukocytes of the indicated genotype were incubated as in panel A. Lysates were probed with antibody that recognizes all SFKs or with anti–phospho-SFK (Y416). (C) WT leukocytes treated with the indicated agent were incubated as in panel A. Lysates were probed with antibody that recognizes all SFKs or with anti–phospho-SFK (Y416). (D) WT leukocytes treated with the indicated agent or leukocytes of the indicated genotype were incubated as in panel A. Lysates were probed with antibody that recognizes all SFKs or with anti–phospho-SFK (Y416). (E) WT leukocytes treated with the indicated agent were incubated as in panel A. Lysates were probed with antibody to Syk or to phospho-Syk. (F) Lysates were Western blotted with antibody to Syk or to phospho-Syk. (G) Lysates were immunoprecipitated with antibody to Btk and then Western blotted with the same antibody or with antiphosphotyrosine antibody. (H) Lysates were probed with antibody to p38 or to phospho-p38. The E-selectin density was 400 sites/μm2 for PSGL-1−/−/CD44−/− leukocytes and 200 sites/μm2 for leukocytes of all other genotypes. The data are representative of at least 3 independent experiments.
To examine the contributions of SFKs to Syk activation, we rotated leukocytes on E-selectin and blotted cell lysates with anti–phospho-Syk antibody. The SFK inhibitor PP2, but not its inactive analog PP3, blocked E-selectin activation of Syk in WT cells (Figure 3D). E-selectin activated Syk in Hck−/− and Lyn−/− leukocytes but not in Fgr−/−, Hck−/−/Lyn−/−, or Fgr−/−/Hck−/−/Lyn−/− cells. Thus, Hck/Lyn as well as Fgr is required to activate Syk. Disrupting lipid rafts blocked Syk activation in WT cells (Figure 3E), consistent with the requirement for rafts to activate SFKs.
E-selectin engagement of PSGL-1 or CD44 sequentially activates Syk, Btk, and p38
Our results in Figures 1 and 2 defined Btk as an essential signaling component for slow rolling upon engagement of PSGL-1 or CD44. To define the sequence of activation of Btk relative to other kinases, we rotated murine leukocytes on E-selectin, then lysed the cells and measured phosphorylation of Syk, Btk, and p38. E-selectin activated Syk, Btk, and p38 in WT, PSGL-1−/−, or CD44−/− neutrophils but not in PSGL-1−/−/CD44−/− neutrophils even at higher density (Figure 3F). E-selectin activated Syk in Btk−/− leukocytes (Figure 3F). Inhibiting Syk blocked activation of both Btk and p38 in WT cells (Figure 3G-H). Inhibiting p38 did not prevent Btk activation in WT cells (Figure 3G). However, E-selectin did not activate p38 in Btk−/− cells (Figure 3H). These data demonstrate that E-selectin engagement of PSGL-1 or CD44 sequentially activates Syk, Btk, and p38.
E-selectin engagement of PSGL-1 requires its cytoplasmic domain to trigger slow rolling and to activate SFKs
To determine whether PSGL-1 requires its cytoplasmic domain to initiate E-selectin–mediated slow rolling, we crossed “ΔCD” mice, which express PSGL-1 without the cytoplasmic domain,19 with CD44−/− mice to eliminate the contribution of CD44 to E-selectin signaling. The surface density of ΔCD PSGL-1 is reduced to approximately 10% of WT levels because of inefficient export from the endoplasmic reticulum.19 To compensate for this difference in density, we perfused ΔCD PSGL-1/CD44−/− leukocytes over a 2-fold higher E-selectin density (400 sites/μm2) to match the mean rolling velocity with that of CD44−/− neutrophils rolling on E-selectin at 200 sites/μm2 (Figure 4A). CD44−/− but not ΔCD PSGL-1/CD44−/− leukocytes rolled slower on coimmobilized ICAM-1. As a second approach, we digested CD44−/− leukocytes with O-sialoglycoprotein endopeptidase (OSGE) to reduce the density of PSGL-1 to the level on ΔCD PSGL-1/CD44−/− leukocytes (Figure 4A inset). OSGE treatment did not remove L-selectin, αLβ2, αMβ2, or CD44 from WT leukocytes (data not shown). OSGE-treated CD44−/− neutrophils and ΔCD PSGL-1/CD44−/− neutrophils rolled with similar velocities on E-selectin, providing functional evidence that OSGE removed E-selectin binding sites on PSGL-1 to the level on ΔCD PSGL-1 leukocytes (Figure 4A). OSGE-treated CD44−/− neutrophils but not ΔCD PSGL-1/CD44−/− neutrophils rolled slower on coimmobilized ICAM-1 (Figure 4A). OSGE treatment of PSGL-1−/− neutrophils did not alter rolling on E-selectin with or without coimmobilized ICAM-1, suggesting that the enzyme's functional effects were limited to PSGL-1 (Figure 4B). These complementary data demonstrate that PSGL-1 requires its cytoplasmic domain to trigger E-selectin–mediated slow rolling.
E-selectin engagement of PSGL-1 requires its cytoplasmic domain to trigger slow rolling and to activate SFKs. (A) Velocities of CD44−/− neutrophils, OSGE-treated CD44−/− neutrophils, or ΔCD PSGL-1/CD44−/− neutrophils rolling on E-selectin at the indicated density with or without coimmobilized ICAM-1 (240 sites/μm2). The inset shows the expression levels of PSGL-1 on cells from the indicated genotype as measured by flow cytometry. (B) Velocities of mock- or OSGE-treated PSGL-1−/− neutrophils rolling on E-selectin (200 sites/μm2) with or without coimmobilized ICAM-1 (240 sites/μm2). (C) OSGE-treated CD44−/− neutrophils or ΔCD PSGL-1/CD44−/− neutrophils were rotated on E-selectin–IgM (400 sites/μm2) in the presence or absence of EDTA or on control CD45-IgM for 5 minutes. Lysates were Western blotted with antibody that recognizes all SFKs or with anti–phospho-SFK (Y416). The data are representative of at least 3 independent experiments. *P < .01.
E-selectin engagement of PSGL-1 requires its cytoplasmic domain to trigger slow rolling and to activate SFKs. (A) Velocities of CD44−/− neutrophils, OSGE-treated CD44−/− neutrophils, or ΔCD PSGL-1/CD44−/− neutrophils rolling on E-selectin at the indicated density with or without coimmobilized ICAM-1 (240 sites/μm2). The inset shows the expression levels of PSGL-1 on cells from the indicated genotype as measured by flow cytometry. (B) Velocities of mock- or OSGE-treated PSGL-1−/− neutrophils rolling on E-selectin (200 sites/μm2) with or without coimmobilized ICAM-1 (240 sites/μm2). (C) OSGE-treated CD44−/− neutrophils or ΔCD PSGL-1/CD44−/− neutrophils were rotated on E-selectin–IgM (400 sites/μm2) in the presence or absence of EDTA or on control CD45-IgM for 5 minutes. Lysates were Western blotted with antibody that recognizes all SFKs or with anti–phospho-SFK (Y416). The data are representative of at least 3 independent experiments. *P < .01.
We plated OSGE-treated CD44−/− neutrophils or ΔCD PSGL-1/CD44−/− neutrophils on E-selectin and measured SFK activity in cell lysates. E-selectin activated SFKs in OSGE-treated CD44−/− neutrophils but not in ΔCD PSGL-1/CD44−/− neutrophils (Figure 4C). These findings demonstrate that E-selectin engagement of PSGL-1 requires its cytoplasmic domain to activate SFKs, the earliest measurable signaling event.
E-selectin engagement of PSGL-1 or CD44 signals slow rolling in vivo
To determine the contributions of PSGL-1 and CD44 to E-selectin–mediated slow rolling in vivo, we measured leukocyte rolling in venules of the cremaster muscle 4 hours after stimulation with TNF-α, which induces expression of P- and E-selectin.9 Hemodynamic and microvascular parameters were equivalent in venules of all genotypes and treatment groups (supplemental Table 1). Rolling velocities were measured in the same venules before and after sequential injection of blocking mAbs to P-selectin and β2-integrins. In WT mice, only mAb to β2-integrins increased rolling velocities (Figure 5A), consistent with the dominant roles of E-selectin and β2-integrins in controlling rolling velocities.8-10 Similar integrin-dependent slow rolling was observed in PSGL-1−/− or CD44−/− mice. In contrast, rolling velocities in PSGL-1−/−/CD44−/− mice were much faster and were not increased by blocking β2-integrins (Figure 5A).
E-selectin–mediated slow rolling in vivo requires PSGL-1 and CD44, all 3 SFKs, and Btk. (A) Velocities of leukocytes rolling in TNF-α–stimulated venules of mice of the indicated genotypes, measured before and after injecting a blocking mAb to P-selectin and then a blocking mAb to β2-integrins. (B-D) Velocities of PKH26- and PKH67-labeled leukocytes of the indicated genotypes in the same TNF-α–stimulated venules. The labeled cells were pretreated with PTx, and the mice were pretreated with anti–P-selectin mAb. (E) Lethally irradiated WT mice were injected with WT LysM-GFP+ bone marrow cells mixed with an equal number of GFP-negative WT or Btk−/− cells. After 8 weeks, the mice were treated with PTx and anti–P-selectin mAb, and rolling velocities of GFP-positive and GFP-negative leukocytes were measured in the same TNF-α–stimulated venules. The data represent the mean ± SEM from at least 3 experiments. *P < .01.
E-selectin–mediated slow rolling in vivo requires PSGL-1 and CD44, all 3 SFKs, and Btk. (A) Velocities of leukocytes rolling in TNF-α–stimulated venules of mice of the indicated genotypes, measured before and after injecting a blocking mAb to P-selectin and then a blocking mAb to β2-integrins. (B-D) Velocities of PKH26- and PKH67-labeled leukocytes of the indicated genotypes in the same TNF-α–stimulated venules. The labeled cells were pretreated with PTx, and the mice were pretreated with anti–P-selectin mAb. (E) Lethally irradiated WT mice were injected with WT LysM-GFP+ bone marrow cells mixed with an equal number of GFP-negative WT or Btk−/− cells. After 8 weeks, the mice were treated with PTx and anti–P-selectin mAb, and rolling velocities of GFP-positive and GFP-negative leukocytes were measured in the same TNF-α–stimulated venules. The data represent the mean ± SEM from at least 3 experiments. *P < .01.
To directly compare the contributions of PSGL-1 and CD44 to slow rolling in the same venules, we labeled WT, PSGL-1−/−, CD44−/−, or PSGL-1−/−/CD44−/− leukocytes with red or green fluorescent dyes. The labeling procedure did not alter rolling velocities on E-selectin alone or αLβ2-dependent slow rolling on E-selectin and ICAM-1 (supplemental Figure 7). We injected a 1:1 mixture of red and green leukocytes intravenously into recipient WT mice 4 hours after injecting TNF-α into the cremaster muscle. Cells were treated with pertussis toxin (PTx) to block chemokine signaling through Gαi-coupled receptors, and mice were injected with anti–P-selectin mAb to ensure that leukocytes rolled only on E-selectin. Compared with WT leukocytes, PSGL-1−/− or CD44−/− leukocytes rolled with similar velocities before and after blocking β2-integrins (Figure 5B). PSGL-1−/−/CD44−/− leukocytes rolled faster than WT leukocytes, and blocking β2-integrins did not alter their velocities (Figure 5B). These data demonstrate that E-selectin engagement of PSGL-1 or CD44 signals integrin-dependent slow rolling in vivo.
Neutrophils use Hck and Lyn as well as Fgr to signal slow rolling on E-selectin in vivo
We labeled WT leukocytes with a red dye and leukocytes lacking 1 or more SFKs with a green dye and injected a 1:1 mixture of PTx-pretreated red and green leukocytes intravenously into recipient WT mice 4 hours after injecting TNF-α into the cremaster muscle and immediately after injecting anti–P-selectin mAb. We compared rolling velocities of WT and SFK-deficient leukocytes in the same venules. WT and Hck−/− leukocytes rolled with similar slow velocities, which increased after injecting anti–β2-integrin mAb (Figure 5C). Compared with WT leukocytes, Fgr−/−, Hck/Lyn−/−, and Fgr−/−/Hck−/−/Lyn−/− leukocytes rolled significantly faster, and their velocities did not further increase after injecting anti–β2-integrin mAb. These data confirm that SFKs other than Fgr make essential contributions to slow rolling in vivo as well as in vitro.
Btk mediates E-selectin–mediated slow rolling in vivo
To test whether Btk also makes important contributions to E-selectin–mediated slow rolling in vivo, we first visualized leukocyte rolling in TNF-α–stimulated venules of Btk−/− mice. The velocities were similar to those of WT leukocytes after blocking β2-integrins, and injecting anti–β2-integrin mAb did not increase their velocities (Figure 5A). To test more precisely the contribution of Btk to slow rolling, we compared rolling velocities of WT and Btk−/− leukocytes in the same TNF-α–stimulated venules. For these studies, we injected equal numbers of PTx-pretreated WT or Btk−/− cells labeled with red or green dye, respectively, and measured rolling velocities in mice after injecting anti–P-selectin mAb. Btk−/− leukocytes rolled significantly faster than WT leukocytes, and injecting anti–β2-integrin mAb did not increase their velocities (Figure 5D). In an alternative approach, we injected irradiated WT mice with WT LysM–green fluorescent protein–positive (GFP+) bone marrow cells (which express GFP in myeloid cells) mixed with an equal number of GFP-negative WT or Btk−/− cells. After reconstitution, we measured leukocyte rolling velocities in TNF-α–challenged venules after injecting mAb to block P-selectin and PTx to block chemokine signaling. The rolling velocities of GFP-positive and GFP-negative WT cells were equivalent, whereas Btk−/− leukocytes rolled significantly faster than WT cells (Figure 5E). These data confirm our in vitro results and establish an important role for Btk in E-selectin–mediated slow rolling in vivo.
Both PSGL-1 and CD44 contribute to Gαi-independent neutrophil recruitment in vivo
Neutrophil migration into the peritoneum after thioglycollate injection is mediated by selectins and chemokines.31,32 To explore the chemokine-independent contributions of PSGL-1 and CD44 in this model, we measured neutrophil recruitment into the peritoneum 4 hours after thioglycollate challenge in WT, PSGL-1−/−, CD44−/−, or PSGL-1−/−/CD44−/− mice pretreated with PTx to inhibit Gαi signaling. Neutrophil influx in PSGL-1−/− mice was reduced by approximately 50%. Recruitment was comparable in WT and CD44−/− mice but was decreased by approximately 90% in PSGL-1−/−/CD44−/− mice (Figure 6A). These data demonstrate that both CD44 and PSGL-1 mediate Gαi-independent neutrophil recruitment in vivo.
Gαi-independent neutrophil recruitment in vivo requires PSGL-1 and CD44, all 3 SFKs, and Btk. (A) WT, PSGL-1−/−, CD44−/−, or PSGL-1−/−/CD44−/− mice received 4 μg of PTx intravenously 2 hours before injection with 1 mL of 4% thioglycollate intraperitoneally. After 4 hours, peritoneal cells were collected, and the number of neutrophils was measured by flow cytometry. (B) WT leukocytes were labeled with PKH67 or PKH26. SFK-deficient leukocytes were labeled with PKH26. Untreated or PTx-treated WT mice were injected intraperitoneally with thioglycollate. After 2 hours, they were injected intravenously with a 1:1 mixture of PKH26- and PKH67-labeled leukocytes. After another 2 hours, blood and peritoneal cells were collected, and the number of neutrophils labeled with each dye was measured by flow cytometry. Results are plotted as the ratio of PKH26-labeled neutrophils from the indicated genotype to PKH67-labeled WT neutrophils. (C) Untreated or PTx-pretreated WT or Btk−/− mice were injected with thioglycollate intraperitoneally. After 4 hours, peritoneal cells were collected, and the number of neutrophils was measured by flow cytometry. (D) Competitive homing was measured as in panel B, except that Btk−/− leukocytes were used instead of SFK-deficient leukocytes. The data represent the mean ± SEM from at least 3 experiments. *P < .01.
Gαi-independent neutrophil recruitment in vivo requires PSGL-1 and CD44, all 3 SFKs, and Btk. (A) WT, PSGL-1−/−, CD44−/−, or PSGL-1−/−/CD44−/− mice received 4 μg of PTx intravenously 2 hours before injection with 1 mL of 4% thioglycollate intraperitoneally. After 4 hours, peritoneal cells were collected, and the number of neutrophils was measured by flow cytometry. (B) WT leukocytes were labeled with PKH67 or PKH26. SFK-deficient leukocytes were labeled with PKH26. Untreated or PTx-treated WT mice were injected intraperitoneally with thioglycollate. After 2 hours, they were injected intravenously with a 1:1 mixture of PKH26- and PKH67-labeled leukocytes. After another 2 hours, blood and peritoneal cells were collected, and the number of neutrophils labeled with each dye was measured by flow cytometry. Results are plotted as the ratio of PKH26-labeled neutrophils from the indicated genotype to PKH67-labeled WT neutrophils. (C) Untreated or PTx-pretreated WT or Btk−/− mice were injected with thioglycollate intraperitoneally. After 4 hours, peritoneal cells were collected, and the number of neutrophils was measured by flow cytometry. (D) Competitive homing was measured as in panel B, except that Btk−/− leukocytes were used instead of SFK-deficient leukocytes. The data represent the mean ± SEM from at least 3 experiments. *P < .01.
Hck and Lyn as well as Fgr mediate Gαi-independent neutrophil recruitment in vivo
To compare migration of WT and SFK-deficient leukocytes in the same mouse, we used a competitive recruitment assay. WT leukocytes labeled with a red dye were mixed with an equal number of WT or SFK-deficient leukocytes labeled with a green dye. Some cell mixtures and recipient mice were pretreated with PTx to inhibit Gαi signaling. The cell mixture was injected intravenously into WT mice 2 hours after intraperitoneal injection of thioglycollate. After another 2 hours, a blood sample was obtained and peritoneal cells were collected. Neutrophils were counted for red or green fluorescence. The ratio of the labeled populations in blood remained close to 1 (Figure 6B). Without PTx pretreatment, equal numbers of WT and SFK-deficient neutrophils entered the peritoneum. With PTx pretreatment, migration of WT and Hck−/− neutrophils was equivalent, whereas migration of Fgr−/−, Hck−/−/Lyn−/−, and Fgr−/−/Hck−/−/Lyn−/− neutrophils was markedly decreased (Figure 6B). Thus, both Hck/Lyn and Fgr make important contributions to Gαi-independent neutrophil recruitment in vivo.
Btk mediates Gαi-independent neutrophil recruitment in vivo
Without PTx pretreatment, neutrophil recruitment to the thioglycollate-challenged peritoneum of WT and Btk−/− mice was comparable. With PTx pretreatment, neutrophil recruitment was reduced by approximately 50% in WT mice but was nearly eliminated in Btk−/− mice (Figure 6C). We used the competitive recruitment assay to compare migration of WT and Btk−/− leukocytes in the same mouse. Equal numbers of red WT leukocytes and green WT or Btk−/− leukocytes were injected intravenously into WT mice 2 hours after intraperitoneal injection of thioglycollate. After another 2 hours, blood and peritoneal cells were collected. Without PTx pretreatment, equal numbers of WT and Btk−/− neutrophils entered the peritoneum (Figure 6D). With PTx pretreatment, migration of Btk−/− neutrophils was reduced by approximately 90%. Thus, Btk is a key contributor to Gαi-independent neutrophil recruitment in vivo.
Discussion
Neutrophils rolling on E-selectin activate integrin αLβ2 to slow rolling on ICAM-1 on endothelial cells. E-selectin signaling activates SFKs, ITAM adaptors, Syk, and p38, and cooperates with chemokine signaling through Gαi-coupled receptors to recruit neutrophils to inflamed tissues.8,15 These earlier studies identified PSGL-1 as the essential E-selectin ligand and Fgr as the only SFK that induce integrin-dependent slow rolling. Here, we demonstrated that neutrophils use both PSGL-1 and CD44 to trigger slow rolling in vitro and in vivo. Either glycoprotein can signal in the absence of the other, through a common pathway that uses the combination of Hck and Lyn as well as a separate role for Fgr to activate Syk (Figure 7). We detected the signaling contributions of Hck and Lyn only in neutrophils lacking both SFKs, which were not examined earlier. Defective signaling in neutrophils lacking only Fgr is consistent with a larger signaling role for Fgr, but Fgr alone could not replace Hck/Lyn. We therefore propose that Hck/Lyn and Fgr have distinct functions. For example, Fgr might phosphorylate the ITAMs of DAP12 and FcRγ, whereas Hck/Lyn might phosphorylate Syk after it docks to the phosphorylated ITAMs.
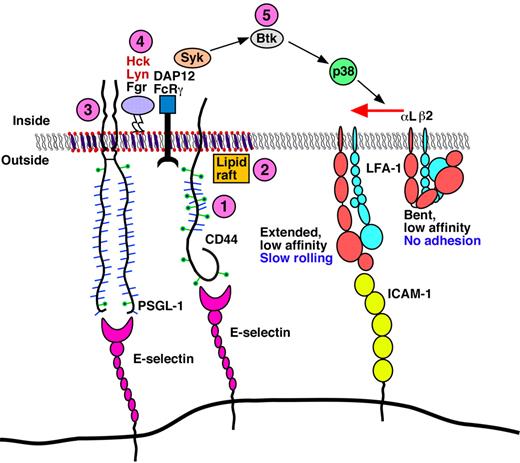
Model for E-selectin–mediated slow rolling. The circled numbers represent new signaling components identified in this paper. Neutrophils rolling on E-selectin engage both CD44 and PSGL-1 to initiate signaling through a common pathway that requires lipid rafts, the cytoplasmic domain of PSGL-1, all 3 SFKs, the ITAM adaptors DAP12 and FcRγ, the Tec kinase Btk, and p38. This signaling cascade activates integrin leukocyte function-associated antigen 1 (LFA-1) to a conformation that enables slow rolling but not arrest on ICAM-1. PSGL-1 and CD44 may not be located in the same raft domains as depicted in the figure.
Model for E-selectin–mediated slow rolling. The circled numbers represent new signaling components identified in this paper. Neutrophils rolling on E-selectin engage both CD44 and PSGL-1 to initiate signaling through a common pathway that requires lipid rafts, the cytoplasmic domain of PSGL-1, all 3 SFKs, the ITAM adaptors DAP12 and FcRγ, the Tec kinase Btk, and p38. This signaling cascade activates integrin leukocyte function-associated antigen 1 (LFA-1) to a conformation that enables slow rolling but not arrest on ICAM-1. PSGL-1 and CD44 may not be located in the same raft domains as depicted in the figure.
Signaling through PSGL-1 or CD44 required intact lipid rafts (Figure 7). This resembles the raft dependence for SFK-mediated signaling through immunoreceptors but not the raft independence for SFK-mediated signaling through integrins.14,22 Raft disruption blocked E-selectin–mediated activation of all 3 SFKs. PSGL-1 and CD44 partition into rafts,19-21 but PSGL-1 is concentrated on microvilli,3,19 whereas CD44 is distributed on the rest of the plasma membrane.33 During rolling, binding of PSGL-1 or CD44 to E-selectin may coalesce small raft domains into larger domains that activate SFKs and position them near ITAM adaptors, Syk, and other substrates. Consistent with this hypothesis, neutrophils rolling on P-selectin recruit Syk into buoyant, detergent-resistant raft fractions.34 We did not confirm a report that disrupting lipid rafts markedly diminishes the number of human neutrophils rolling on P- or E-selectin alone.34 Chelating cholesterol modestly impairs rolling by reducing membrane deformability,35 which hinders extension of adhesive membrane tethers.36 In our experimental system, these effects were manifested by slightly faster rolling velocities and more frequent cell detachments at higher wall shear stresses (3-10 dynes/cm2). However, we consistently observed that disrupting rafts altered neither the numbers nor velocities of human or murine leukocytes rolling on P- or E-selectin at conventional wall shear stresses (1-2 dynes/cm2).
The cytoplasmic domain is not needed to target PSGL-1 to rafts,19 but it was essential to activate SFKs and induce slow rolling upon E-selectin engagement (Figure 7). Ezrin/radixin/moesin (ERM) proteins bind to the cytoplasmic domains of PSGL-1 and CD44.37,38 Cross-linking PSGL-1 recruits Syk to an atypical ITAM on ERM proteins bound to the cytoplasmic domain of PSGL-1.39 However, this potential signaling mechanism cannot induce slow rolling in the absence of the conventional ITAM adaptors DAP12 and FcRγ. Furthermore, engaging E-selectin does not activate Syk in DAP12−/−/FcRγ−/− neutrophils.15 The actin cytoskeleton regulates clustering of lipid rafts,40,41 and ERM proteins connect the cytoplasmic domains of PSGL-1 and CD44 to the cytoskeleton.37,38,42 These cytoskeletal interactions might promote raft clustering and initiate signaling during rolling on E-selectin.
We identified the Tec kinase Btk as a key intermediate for E-selectin–mediated slow rolling, positioned between Syk and p38. Although neutrophils also express the Tec kinases Tec and Bmx,26 they could not activate p38 or induce slow rolling in the absence of Btk. Neutrophils lacking Tec and/or Bmx would be needed to determine whether these kinases make other contributions to E-selectin signaling. SFKs can activate Tec kinases,43 but inhibiting Syk blocked Btk activation in neutrophils plated on E-selectin, suggesting that Syk directly activates Btk. In some situations, Btk activation is downstream of PI3K, which generates PIP3 at the plasma membrane that recruits Btk through its pleckstrin homology domain.26,28,43 However, neither inhibiting PI3K in WT neutrophils, reducing PI3K activity in PI3Kγ−/− or PI3Kδ−/− neutrophils, nor increasing PIP3 in SHIP1−/− neutrophils altered E-selectin–mediated slow rolling. Instead, Btk may be recruited to the adaptor Src homology 2 domain–containing leukocyte-specific phosphoprotein of 76 kDa as part of a raft-associated signaling complex like those used by immunoreceptors.14
In vitro, the signaling events that P-selectin and E-selectin propagated were indistinguishable. Both selectins activated all 3 SFKs in neutrophils, which does not support the suggestion that P-selectin and E-selectin initiate signaling through PSGL-1 in different ways.15 Inhibiting Syk activity or knocking down Syk mRNA was reported to markedly reduce the number of human myeloid KG1 cells rolling on P-selectin but not on E-selectin.34 However, we observed that inhibiting SFKs, Syk, Tec kinases, or p38 had no effect on the numbers or velocities of human or murine neutrophils rolling on P- or E-selectin alone. Instead, these inhibitors prevented integrin-dependent slow rolling on coimmobilized ICAM-1. Engagement of PSGL-1 can activate other kinases,44-47 but whether they contribute to slow rolling requires further study. In TNF-α–stimulated venules, neutrophils roll faster on P-selectin than on E-selectin even in the absence of β2-integrins.11 Thus, E-selectin governs slow rolling through both integrin-dependent and -independent mechanisms.9 P-selectin–mediated integrin activation may be more important when neutrophils adhere to activated platelets.48,49 The cytoplasmic domain of PSGL-1 binds to Nef-associated protein-1.23 Prolonged binding of antibodies or P-selectin to PSGL-1 activates SFKs that phosphorylate Nef-associated protein-1, providing a docking site for the p85 subunit of PI3Kδ. This propagates downstream signals that activate integrin αMβ2.23 However, only a few selectin-ligand bonds mediate signaling during rolling, and inhibiting PI3K in human or WT murine neutrophils or reducing PI3K activity in PI3Kγ−/− or PI3Kδ−/− neutrophils did not prevent P- or E-selectin–induced slow rolling. Furthermore, the increased PIP3 levels in SHIP1−/− neutrophils did not augment slow rolling on ICAM-1 and enhanced firm adhesion to ICAM-1 only when chemokine was coimmobilized.
In vivo, PSGL-1 has a major role in tethering flowing leukocytes to E-selectin.5 CD44 has been contrasted as an E-selectin ligand that mediates slow rolling, although its potential to activate integrins was not examined.7 We did not confirm the reported faster rolling of CD44−/− than WT neutrophils in TNF-α–activated venules6 ; indeed, this velocity difference was less evident in a second report by the same group.7 Furthermore, WT and CD44−/− neutrophils rolled with similar velocities on E-selectin in vitro, in agreement with ex vivo data.8 By measuring rolling velocities on E-selectin in vivo before and after injecting anti–β2-integrin mAb, we demonstrated that PSGL-1 and CD44 have comparable functions as rolling ligands and as signaling receptors. Both functions cooperate to slow rolling in TNF-α–stimulated venules. PSGL-1 or CD44 might signal in conjunction with E-selectin ligand-1 or an uncharacterized ligand. However, such ligands were not sufficient to trigger integrin-dependent slow rolling of PSGL-1−/−/CD44−/− neutrophils even at high E-selectin densities. By the many criteria that we examined, PSGL-1 and CD44 induce integrin activation through a common pathway. Nevertheless, there is evidence for unique signaling events. Neutrophils rolling in TNF-α–activated venules cluster L-selectin and PSGL-1 at the trailing edge by a p38-dependent mechanism.7 CD44−/− neutrophils do not form these clusters, whereas PSGL-1−/− neutrophils still cluster L-selectin. Thus, although both CD44 and PSGL-1 use p38 to induce integrin-dependent slow rolling, only CD44 induces L-selectin (and PSGL-1) to cluster. Neutrophils rolling in TNF-α–activated venules cluster αMβ2 at the leading edge through SFK-dependent but Syk- and p38-independent signals transduced by E-selectin ligand-1.50
Neutrophils contacting E-selectin serially phosphorylated SFKs, Syk, Btk, and p38 in the absence of ICAM-1, excluding outside-in integrin signaling as a cause for kinase activation. PSGL-1, CD44, all 3 SFKs, and Btk contributed to Gαi-independent neutrophil recruitment to inflamed tissues, underscoring the physiologic relevance of our findings. That PSGL-1 required its cytoplasmic domain to activate SFKs suggests a similar requirement for CD44 and a common mechanism for both proteins to initiate lipid raft–dependent signaling as neutrophils roll on E-selectin. Further study of these events may suggest more precisely targeted anti-inflammatory agents with less toxicity.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Barry Wolitzky and Dietmar Vestweber for antibodies; Gerald Krystal, Clifford Lowell, Klaus Ley, Wasif Khan, Carol Webb, Joseph Pennington, Paul Kubes, and James Ihle for mice; and Mary Beth Humphrey and William Rodgers for reading the paper.
This work was supported by National Institutes of Health grants HL034363 and HL085607.
National Institutes of Health
Authorship
Contribution: T.Y., B.S., J.J.M., L.Y., A.G.K., and K.M. performed research; T.Y., K.M., K.M.C., and R.P.M. analyzed data; and R.P.M. designed research and wrote the paper.
Conflict-of-interest disclosure: R.P.M. has equity interest in Selexys, a start-up company that is developing inhibitors of selectins and selectin ligands. The remaining authors declare no competing financial interests.
The current address for K.M. is Osaka University Dental Hospital, Osaka 565-0871, Japan.
Correspondence: Rodger P. McEver, Cardiovascular Biology Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: rodger-mcever@omrf.org.