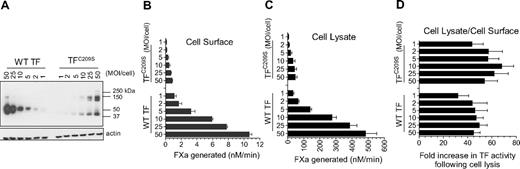
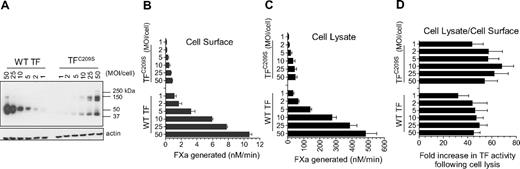
The primary focus of our recent report1 is to examine whether Cys186-Cys209 disulfide–mutated tissue factor (TF) mimics cryptic TF and the importance of Cys186-Cys209 disulfide bond formation in TF decryption, 2 key issues that were not examined earlier.2
In our view, one should be careful not to equate TF mutants that exhibit low procoagulant activity with cryptic TF. This incorrectly broadens the definition of cryptic TF and creates unnecessary confusion and controversy. The classic definition of cryptic TF is that it can form a stable complex with factor VIIa (FVIIa), but the resultant TF-FVIIa complexes fail to activate its macromolecular substrates, factors IX and X.3,4 Therefore, when probing the cryptic nature of TF, one should evaluate the activity of TF-FVIIa complexes at the cell surface. Despite some inherent differences in their binding properties, both cryptic and decrypted TF form stable high-affinity associations with FVII/FVIIa, well below plasma concentrations of FVII (see references in Bach and Monroe5 ). Cryptic TF would remain cryptic even in the presence of supraphysiologic concentrations of FVIIa. TF mutants that exhibit lower procoagulant activity at physiologic concentrations of FVII/FVIIa, but could be rescued by supraphysiologic concentrations of FVIIa should not be equated with cryptic TF. The Cys186-Cys209 disulfide–mutated TF falls into this category. They exhibit low procoagulant activity because the Cys186-Cys209 disulfide bond is required for efficient FVIIa binding1,2 and not because they conform to cryptic TF form.
One of the criticisms of our study raised by Ruf and Versteeg is that in concluding that the allosteric disulfide bond is not essential for TF's procoagulant activity, we took into consideration only data at supraphysiologic concentrations of TF ligands. Because the physiologic concentration of FVIIa was not sufficient to saturate the TF mutants at the cell surface, we had to use higher concentrations of FVIIa. We actually measured the amount of TF-FVIIa complexes formed at the cell surface and used this number to determine the specific activities of wild-type and TF mutants. This experimental design was appropriate and correct to probe the cryptic nature of TF mutants. Nonetheless, to provide a complete picture to the reader, in our report we have also provided data on TF activity measurements in the presence of low concentrations of FVIIa. In their letter, Ruf and Versteeg also suggest that clotting assay is the “gold standard” for TF procoagulant activity. However, the clotting assay alone is not appropriate and useful in investigating the cryptic nature of TF.
Ruf and Versteeg showed that extreme overexpression of TFC209A yielded cell-surface TF procoagulant activity that was similar to cells expressing very low levels of wild-type TF and decryption induced by cell lysis was much more efficient in cells expressing wild-type TF compared with cells expressing TFC209A. These data differ from our recent data obtained using TFC186SC209S mutant.1 In our studies, we did not observe extreme overexpression of TF mutants, as observed by Ruf and Versteeg, when endothelial cells were transduced with 25- to 50-fold higher concentration of virus encoding TF mutants (25-50 MOI/cell) compared with wild-type TF (1-2 MOI/cell) to obtain similar levels of TF expression at the cell surface. Ruf and Versteeg suggest that cotransduction with PAR2 in their study may be responsible for overexpression of TFC209A. However, their own data (see Figure 1B of their letter) fail to support this assumption.
Here, we examined TF decryption induced by cell lysis in wild-type TF and TFC209S mutant (Figure 1). Consistent with the data reported recently1 for TFC186SC209S mutant, we found a similar fold-increase in TF activity (∼ 40- to 50-fold) in cells expressing either TFC209S mutant or wild-type TF upon their lysis (Figure 1D). It is unclear why Ruf and Versteeg failed to obtain similar data. It is possible that extreme overexpression of TFC209A in their study might have resulted in very low TF–anionic phospholipid ratio upon cell lysis compared with wild-type TF, which was expressed at very low levels. It is also possible that TFC209A mutant could have behaved differently from that of our TFC209S mutant. Here it may be pertinent to note that in their earlier studies they found that their TFC209A and TFC186A mutants behaved differently.6 In our studies, we obtained similar data with all 3 of our disulfide mutants: TFC186S, TFC209S, and TFC186C209S. Limited experiments performed with alanine-substituted mutants (plasmid transfection in Chinese hamster ovary cells) also yielded data similar to that of serine mutants.
TFC209S disulfide mutant can be decrypted similar to the wild-type TF. Human umbilical vein endothelial cells cultured in 24-well plate were transduced with varying concentrations (MOI/cell) of adenovirus encoding wild-type (WT) TF or TFC209S. (A) After culturing cells for 48 hours, the cell lysates were harvested, subjected to nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotted for TF using polyclonal anti-TF antibodies. (B) Intact cell monolayers were used to measure cell-surface TF activity by measuring the rate of factor Xa generation after addition of FVIIa (10nM) and factor X (175nM). (C) Cell lysates were made by repeated (3×) freeze-thaw of cell monolayers and diluted 10× before they were used to measure TF activity as in Figure 1B. (D). Fold increase in TF activity upon cell lysis was calculated by the rate of FXa generation in cell lysates divided by the rate of FXa generation at the cell surface. Note: In these experiments, unlike the data shown in our recent report,1 we have not quantified the levels of TF or TF-FVIIa complexes at the cell surface; therefore, one should not extrapolate these data to TF-specific activity.
TFC209S disulfide mutant can be decrypted similar to the wild-type TF. Human umbilical vein endothelial cells cultured in 24-well plate were transduced with varying concentrations (MOI/cell) of adenovirus encoding wild-type (WT) TF or TFC209S. (A) After culturing cells for 48 hours, the cell lysates were harvested, subjected to nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotted for TF using polyclonal anti-TF antibodies. (B) Intact cell monolayers were used to measure cell-surface TF activity by measuring the rate of factor Xa generation after addition of FVIIa (10nM) and factor X (175nM). (C) Cell lysates were made by repeated (3×) freeze-thaw of cell monolayers and diluted 10× before they were used to measure TF activity as in Figure 1B. (D). Fold increase in TF activity upon cell lysis was calculated by the rate of FXa generation in cell lysates divided by the rate of FXa generation at the cell surface. Note: In these experiments, unlike the data shown in our recent report,1 we have not quantified the levels of TF or TF-FVIIa complexes at the cell surface; therefore, one should not extrapolate these data to TF-specific activity.
In conclusion, our recent reports1,7,8 demand candid discussion on recently postulated hypotheses that PDI regulates TF procoagulant activity by its chaperone activity9 and/or Cys186-Cys209 disulfide bond isomerization.6
Authorship
Contribution: H.K. performed the experiments described in this letter and contributed to writing the letter; and L.V.M.R. and U.R.P. designed the research and wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao or Usha Pendurthi, Center for Biomedical Research, The University of Texas Health Science Center at Tyler, UT Health Center at Tyler, 11937 US Hwy 271, Tyler, TX 75708; e-mail: vijay.rao@uhtct.edu or usha.pendurthi@uthct.edu.