The use of warfarin has a well-known bleeding risk. Recombinant activated factor VII (rFVIIa) is a non–plasma-derived, rapid-acting, and rapidly infused potential treatment. This randomized, single-center, placebo-controlled, double-blinded, dose-escalation, exploratory phase 1 trial assessed safety and effects of rFVIIa in reversing warfarin-induced changes in bleeding and coagulation parameters, using a punch biopsy–induced bleeding model in healthy subjects. The effects of warfarin (experiment 1) and rFVIIa (5-80 μg/kg; experiment 2) were evaluated. Outcomes were bleeding duration, blood loss, coagulation parameters, and safety. Warfarin treatment significantly increased bleeding duration and blood loss from pretreatment (experiment 1, 12 subjects). However, these parameters after rFVIIa treatment were not significantly different from placebo (experiment 2, 85 subjects). Mean activated partial thromboplastin time, prothrombin time, and international normalized ratio were reduced from warfarin-elevated levels. rFVIIa (80 μg/kg) significantly reversed warfarin effects on all thromboelastography parameters, compared with placebo (P < .05), and returned the thrombin generation speed to baseline. There were no thromboembolic or serious adverse events. In this exploratory trial, the reversal of warfarin effects was observed in the thromboelastography, thrombin generation, and clotting assays. However, this reversal did not translate to improvements in the bleeding model parameters evaluated in the punch biopsy model. Trial registration is exempt (phase 1).
Introduction
The use of warfarin and other vitamin K antagonists (VKAs) to reduce thrombotic risks in patients with cardiovascular or other diseases has a well-known risk of bleeding,1,2 estimated at 2% to 3% for major hemorrhage1,–3 and 0.6% for fatal bleeding events.3 Current treatment options to reverse the effects of VKA include the administration of vitamin K, coagulation factor replacement (eg, fresh frozen plasma [FFP] or prothrombin complex concentrates), and bypassing therapies (eg, recombinant activated factor VII [rFVIIa]; NovoSeven, Novo Nordisk A/S).1 Among these treatment options, vitamin K, FFP, and prothrombin complex concentrates are licensed for the reversal of VKAs.4,5 These interventions provide either delayed action with long duration reversal or potential rapid action, albeit with short duration. Critical life-threatening bleeding, however, often requires rapid reversal,6 which may not be practical with vitamin K or FFP.1,7 Prothrombin complex concentrates and FFP also have a small risk of transfusion-transmitted infection.8,9 Further, FFP can cause circulatory fluid overload in critically ill patients with severe bleeding, which is of particular concern in certain populations at risk, such as the elderly and pediatric populations.10
rFVIIa is licensed for the treatment of bleeding in patients with hemophilia with inhibitors; however, its ability to mitigate bleeding secondary to VKAs has been limited to demonstrations in several case studies investigating the use of rFVIIa in these clinical situations.11,,,,,,,,,–21 rFVIIa is a non–plasma-derived, rapid-acting, and rapidly infused potential alternative treatment for such bleeding.1 In the available series, treatment with rFVIIa has been found to be generally effective and safe, although one case of purported disseminated intravascular coagulation has been noted.20 The present report is the first prospectively designed exploratory study to evaluate the effects of rFVIIa both in vivo and ex vivo, with an attempt to define an optimal dose needed to control hemorrhage with the use of appropriate controls. Previous studies have mostly evaluated warfarin reversal with the use of only ex vivo clotting assays (eg, activated partial thromboplastin time [aPTT], prothrombin time [PT], and international normalized ratio [INR])15,17,,–20 and did not directly evaluate the effects of rFVIIa either through global hemostatic measurement of bleeding or coagulation profiles through thromboelastography (TEG) or thrombin generation (TG) assays. Nevertheless, these methods have never been systematically characterized in a controlled setting. The current study attempts to combine these approaches to provide a better understanding of the role of rFVIIa in reversing the effects of VKAs.
The current concept of coagulation proposes that it occurs in 3 stages (ie, initiation, amplification, and propagation) involving a series of well-described interactions between coagulation factors on the surface of tissue factor (TF)–bearing cells and activated platelets.22,–24 At pharmacologic doses, rFVIIa can directly activate factor X on the surfaces of activated platelets, leading to the formation of a stable hemostatic plug at the site of injury.24 Clotting assays (eg, aPTT, PT, and INR) have shown limited correlation to bleeding, because they are ex vivo assessments performed in citrated plasma lacking activated platelets.25,,–28 Further, they only describe the initial stage of coagulation. In contrast, TEG can provide continuous coagulation profiles of clot formation in whole blood from initiation to final clot strength, whereas TG can determine the ability to generate the central coagulation enzyme, thrombin.29 It has also been suggested that TEG and TG could be useful ex vivo tests, because they mimic the coagulation processes in vivo.30,31 These assays are currently used to monitor clot dynamics with factor replacement or bypassing treatment and to provide insight into the kinetics of drug mechanisms.30,,,,,–36 However, because of the reported sensitivity of these tests to conditions such as tissue factor concentrations, coagulation activator concentrations, and intersubject variability, their applicability in assessing treatments such as rFVIIa is inconclusive.31,37 Nevertheless, TG and TEG have shown promise as tools for predicting clinical response in coagulation therapy,31,37 and by carefully controlling the experimental conditions in the current study, it was our expectation that the use of TEG and TG assays in this trial would provide further insights into the coagulation process than possible from PT, aPTT, and INR assays alone.
Tests of bleeding time such as the Ivy or Simplate tests yield highly variable bleeding times in healthy subjects.38,39 The punch biopsy model used in the current trial represents a significant conceptual improvement over these tests. This model has produced consistent bleeding times and blood loss in a prior trial involving healthy subjects not treated with VKAs (F7DRC-2157, Novo Nordisk; Recombinant activated factor VIIa dose-escalation trial: Safety in healthy volunteers using a punch biopsy bleeding model. D. Gabriel, H. Roberts, D. Monroe, J. Rand, A.E.P., and B.E.S., manuscript in preparation). Furthermore, the bleeding durations observed were sufficiently prolonged to allow the detection of treatment differences. However, the model was unsuccessful in showing an effect of rFVIIa on bleeding parameters, which may have been the result of the study being performed in a normal coagulation system. It was our expectation that the effect of rFVIIa might be better characterized in the setting of an impaired coagulation system, such as those of subjects treated with VKA.
The current exploratory trial evaluated the effect of rFVIIa in reversing warfarin-induced changes in coagulation parameters and, more importantly, the biologic action of rFVIIa in the cessation of bleeding in a carefully controlled experimental model that used the punch biopsy technique. The study involved in vivo assessments of bleeding (bleeding duration and blood loss), ex vivo whole blood coagulation profiles (TEG), TG, and standard clotting assays (INR, PT, and aPTT) with appropriate controls. The objectives were to initially show that the punch biopsy bleeding model could produce significantly increased bleeding duration and blood loss in subjects treated with warfarin and then to evaluate the effect of rFVIIa in reversing such warfarin-induced bleeding.
Methods
Study design and ethics
This was a randomized, single-center, placebo-controlled, double-blinded, dose-escalation exploratory trial to assess the effects and safety of rFVIIa treatment of punch biopsy–induced bleeding in healthy subjects receiving warfarin. The first part of the trial (experiment 1) evaluated the effects of warfarin on bleeding and coagulation characteristics, and experiment 2 evaluated the effects of rFVIIa (in a series of increasing doses from 5, 10, 20, 40, and 80 μg/kg) on reversing the effects of warfarin on these measures. This trial was conducted in accordance with the Declaration of Helsinki40 and its amendments in force at the time of trial initiation. It was also reviewed and approved by the Heartland Institutional Review Board. Written informed consent was obtained from each subject.
Study population and treatment
The trial was conducted from January 2007 to July 2008 at a phase 1 research center. Healthy men aged 18 to 45 years with normal INR (< 1.2) who met rigorous cardiovascular criteria (eg, normal levels of high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein a) were included in the trial.41 Persons of African American ethnicity were excluded because they were observed to have high intersubject variability in bleeding duration in a previous punch biopsy study (F7DRC-2157, Novo Nordisk), and persons taking investigational drugs, receiving anticoagulation therapy or vitamin K, or planning to undergo surgery were excluded. All subjects in both experiments received 5.0 to 10.0-mg daily oral doses of warfarin sodium (Coumadin; Bristol-Myers Squibb Co) and were titrated to an INR of 2.5 over 2 weeks on an outpatient basis. Subjects in experiment 2 were randomly assigned 6:2 (or 6:6; please see “Statistical analysis” for details) to receive a single intravenous dose of rFVIIa or placebo, which were supplied as identical freeze-dried powder to be reconstituted with sterile water for injection. Subjects were assigned to the lowest available randomization number determined by a randomization schedule provided by the sponsor (Novo Nordisk) by an independent pharmacist.
The punch biopsy was performed on the back of the thigh (locally anesthetized with lidocaine without epinephrine) to a subcutaneous depth of 4 to 6 mm with the use of a Disposable Biopsy Punch (Shoney Scientific) with a diameter of 5 mm. Subjects in experiment 1 underwent 2 biopsies: one before warfarin administration (biopsy B0) and one biopsy after achieving a stable INR of 2.5 or greater (biopsy B1). Subjects in experiment 2 underwent 4 biopsies: one before warfarin administration (B0), one after achieving a stable INR of 2.5 or greater (B1), one at 13 minutes after administration of the trial product (rFVIIa or placebo; biopsy B2; trial product was given 2 hours after B1) and one 5 hours later (∼ 2 T1/2 of rFVIIa; biopsy B3). Subjects could be withdrawn if their bleeding at B0 exceeded 25 minutes or if their bleeding duration at B1 was less than 1.4 times that at B0 (B1/B0 < 1.4), indicating a limited warfarin effect. A stable INR was characterized as a change in INR of less than 0.3 over 2 consecutive measurements separated by a minimum of 2 days.
Efficacy and safety evaluations
Bleeding duration, volume of blood loss, clot dynamics (TEG), TG, and standard coagulation assays (INR, PT, aPTT) were evaluated at each biopsy. The primary end point was the punch biopsy–induced bleeding duration after rFVIIa administration (B2). Bleeding duration was defined as the time from blood emergence to the cessation of bleeding. Secondary end points included the volume of blood loss and clot dynamics as measured by TEG. The volume of blood loss was derived from a conversion (by 1.05 g/mL) of the weight of blood on preweighed filter paper. Clot dynamics were evaluated with the use of the TEG Hemostasis System (Haemoscope Corporation) with citrated blood. Clotting was initiated with TF (Innovin; Dade Behring) diluted to a final dilution of 1:47 000 TF to increase the sensitivity of the TEG assay.31,42 Standard TEG parameters were evaluated.
Thrombin generation, clotting assays (INR, aPTT, and PT [in both experiments]) and coagulation factors II, VII, IX, and X; Protein C; and Protein S (Experiment 1 only) were also evaluated. Thrombin generation was assayed with the use of the Calibrated Automatic Thrombin Generation Assay (Thrombinoscope BVF), with platelet-poor plasma. The manufacturer's low TF reagent was used to increase TG sensitivity. Subject safety was continuously monitored during the trial, with particular attention paid to thromboembolic events and troponin-I levels, which were assayed with the Advia Centaur Cardiac Troponin Kit. A full safety assessment was conducted at the end of the 20-, 40-, and 80-μg/kg rFVIIa dose tiers.
Statistical analyses
The null hypothesis was that the bleeding duration at B2 after rFVIIa treatment was greater than or equal to that with placebo, and the alternative hypothesis was that bleeding duration after rFVIIa treatment was less than that with placebo. All subjects who received warfarin (experiment 1) and warfarin and rFVIIa or placebo (experiment 2) were included in the safety analyses. All randomly assigned subjects who underwent B0 and B1 were included in the intent-to-treat (ITT) analysis set.
Sample size calculation and adaptive design.
The planned sample size (10-12 subjects) in experiment 1 was not based on formal sample size calculations. The planned sample size in experiment 2 was 36 to 84 subjects and was based on an adaptive study design that involved systematic evaluation at each rFVIIa dose tier and allowed for smaller sample sizes while allowing a quicker determination of the optimally effective rFVIIa dose, compared with a conventional study design. The maximum sample size of 24 allowed in the adaptive design for the highest dose group and placebo group was sufficient to detect with a power of 80% the mean difference in bleeding duration between the groups as small as 10% of the placebo value at a significance level of .05. Experiment 2 started with 8 subjects (6 rFVIIa; 2 placebo) in the 5-μg/kg dose tier and progressed in ascending dose tiers up to 80 μg/kg. Interim analyses were performed at the 3 highest dose tiers (20, 40, and 80 μg/kg), in which normalization of the warfarin-induced bleeding duration was assessed (defined as the ratio of bleeding at B2/B0 of ≤ 1.1). Normalization was not achieved at any dose tier in this trial, and the trial was continued to the highest dose tier. Because comparisons between rFVIIa treatment and placebo required the number of placebo-treated subjects to be not less than that of the rFVIIa-treated subjects, a second cohort for the 80-μg/kg dose tier was randomized, as planned, in a ratio of 6:6 (rFVIIa/placebo) to fulfil this requirement.
Efficacy and safety analyses.
The primary efficacy analysis was conducted with the use of a generalized linear model with logarithmic link and γ distribution, with treatment group as a fixed factor and the log-transformed bleeding duration at B1 as a covariate. Identical analyses were performed for blood loss. Other secondary end points were presented with descriptive statistics. Because of the unexpected closure of the research facilities responsible for performing the TEG and TG evaluations, TG data were available only for the 80-μg/kg dose tier. Limited data availability for the TEG and TG resulted in formal analyses being conducted for only the 80-μg/kg dose tier.
Results
Subject disposition and demographics
Experiment 1.
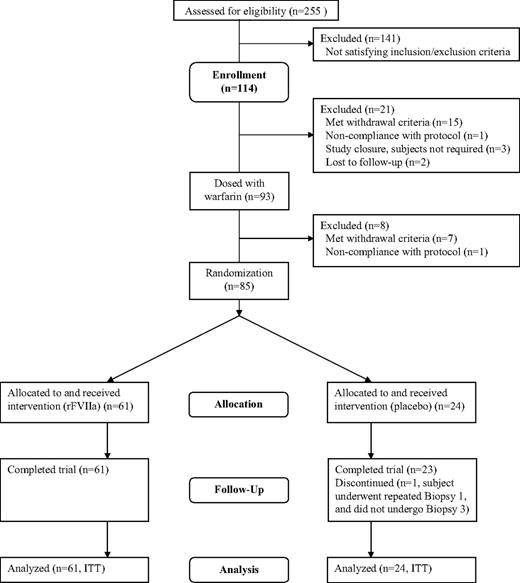
Twelve subjects fulfilled the screening criteria and had a baseline biopsy followed by warfarin exposure to achieve a targeted INR of 2.5 (± 0.3). All 12 subjects were included in the safety analyses. One subject was withdrawn before B1, having failed to achieve a qualifying INR level. The ITT analysis set comprised 11 subjects.
Experiment 2.
A total of 114 subjects fulfilled the screening criteria and enrolled in the trial. The safety analysis and ITT analysis sets comprised 85 subjects (Figure 1). One placebo-treated subject required a repeat of B1 and was thus withdrawn from the trial without completing B3, after reaching the biopsy limit of 4 after B2; however, he was included in the ITT analysis because he had undergone the first 2 biopsies. A total of 8 subjects treated with warfarin were withdrawn from the trial: 6 due to the withdrawal criterion of B1/B0 ratio less than 1.4, 1 due to voluntary withdrawal of consent, and 1 due to noncompliance with the protocol (positive urine drug test).
Demographic variables and other baseline characteristics of subjects in the ITT population in both experiments are presented in Table 1. Most of the subjects were white. The mean (± SD) subject age ranged from 25.3 (± 6.9) to 30.0 (± 10.2) years, and body mass index ranged from 24.2 (± 5.0) to 26.7 (± 4.2) kg/m2 across treatment groups.
Bleeding duration and blood loss
Effect of warfarin.
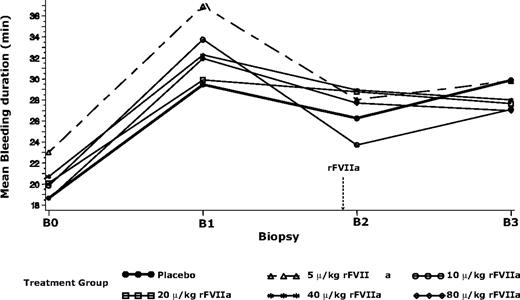
Bleeding duration and volume of blood loss in all treatment groups were greater after warfarin treatment, compared with baseline (Table 2; Figure 2). Bleeding duration (mean ± SD) was increased by 7.9 minutes in experiment 1 (P < .001), and similarly by 9.8 to 13.9 minutes (rFVIIa groups) and 10.8 minutes (placebo group) in experiment 2. Blood loss was significantly increased by 1.5 mL in experiment 1 (P < .001) and by 0.4 to 4.8 mL (rFVIIa group) and 1.6 mL (placebo group) in experiment 2. The large standard deviation in the baseline bleeding duration of the 5-μg/kg group was indicative of somewhat higher variability in biopsy technique in the first of the dose groups.
Mean bleeding duration before and after warfarin and trial product (rFVIIa or placebo) treatments for subjects in experiment 2. B0 indicates biopsy 0 (before warfarin treatment); B1, biopsy 1 (after warfarin treatment to achiever INR of 2.5 ± 0.3); B2, biopsy 2 (15 minutes after rFVIIa or placebo treatment); B3, biopsy 3 (5 hours after B2). Dashed arrow indicates time of rFVIIa administration. The model used was a generalized linear model with logarithmic link and γ distribution, and bleeding duration at B1 as a covariate. Mean values for B0 and B1 were geometric means, and mean values for B2 and B3 were least-square means derived from the generalized linear model.
Mean bleeding duration before and after warfarin and trial product (rFVIIa or placebo) treatments for subjects in experiment 2. B0 indicates biopsy 0 (before warfarin treatment); B1, biopsy 1 (after warfarin treatment to achiever INR of 2.5 ± 0.3); B2, biopsy 2 (15 minutes after rFVIIa or placebo treatment); B3, biopsy 3 (5 hours after B2). Dashed arrow indicates time of rFVIIa administration. The model used was a generalized linear model with logarithmic link and γ distribution, and bleeding duration at B1 as a covariate. Mean values for B0 and B1 were geometric means, and mean values for B2 and B3 were least-square means derived from the generalized linear model.
Effect of rFVIIa.
Mean bleeding duration was changed from warfarin-elevated levels after treatment with rFVIIa in all dose groups (Table 2; Figure 2). None of these changes were statistically significant. Treatment with increasing doses of rFVIIa did not result in consistent changes in blood loss from warfarin-elevated levels (Table 2). The baseline-adjusted least square bleeding duration and blood loss after rFVIIa treatment (at B2) were not significantly different from placebo (P > .05; Table 2).
Effect of warfarin and rFVIIa on coagulation characteristics
Coagulation factors (experiment 1).
The levels of coagulation factors were all significantly reduced below their normal ranges after warfarin treatment, from 108.4% to 31.0% (factor II), 81.8% to 7.6% (factor VII [FVII] activity), 111.0% to 22.6% (coagulant activity of FVII), 80.6% to 23.8% (factor IX), and 109.0% to 13.8% (factor X [FX]) (P < .001). The levels of anticoagulants were also reduced, from 104.4% to 36.9% (protein C) and from 136.3% to 64.4% (protein S) (P < .001).
aPTT, PT, and INR (experiments 1 and 2).
Mean aPTTs, PTs, and INRs were increased beyond the normal range after warfarin treatment and were reduced after treatment with rFVIIa (Table 3). Although the mean aPTTs, PTs, and INRs were brought into the normal range in the higher dose groups, they remained above the mean baseline level. Mean INRs shortly after rFVIIa administration (at B2) were significantly lower in all rFVIIa dose groups (1.5, 1.3, 1.2, 1.2, and 1.2 in the 5- to 80-μg/kg groups, respectively), compared with the placebo group (2.5) (P < .001). Similar observations were made for the mean aPTTs and PTs.
Thrombin generation (experiment 2).
TG data were only analyzed for the 80-μg/kg rFVIIa and placebo groups. Treatment with warfarin resulted in substantial increases from baseline in lag time and time to maximum concentration and decreases in maximum concentration and endogenous thrombin potential in both the placebo and the 80-μg/kg rFVIIa treatment groups (Figure 3). Treatment with rFVIIa returned some TG parameters to baseline level (lag time) or close to baseline levels (time to maximum concentration). These effects were sustained up to the last measured time point of 180 minutes after rFVIIa administration. Maximum concentration and endogenous thrombin potential remained unchanged. Treatment with placebo resulted in negligible changes in these parameters.
TG parameters in the placebo or 80-μg/kg groups in experiment 2. TG parameters in subjects treated with placebo or 80 μg/kg rFVIIa at (A) lag time, (B) time to maximum concentration, (C) maximum concentration, and (D) endogenous thrombin potential. B0 indicates biopsy 0 (before warfarin treatment); B1, biopsy 1 (after warfarin treatment to achiever INR of 2.5 ± 0.3); B2, biopsy 2 (15 minutes after rFVIIa or placebo treatment). Arrow indicates time of rFVIIa administration.
TG parameters in the placebo or 80-μg/kg groups in experiment 2. TG parameters in subjects treated with placebo or 80 μg/kg rFVIIa at (A) lag time, (B) time to maximum concentration, (C) maximum concentration, and (D) endogenous thrombin potential. B0 indicates biopsy 0 (before warfarin treatment); B1, biopsy 1 (after warfarin treatment to achiever INR of 2.5 ± 0.3); B2, biopsy 2 (15 minutes after rFVIIa or placebo treatment). Arrow indicates time of rFVIIa administration.
Thromboelastography (experiment 2).
TEG data are only presented for the 80-μg/kg and placebo groups for consistency with the TG data. Treatment with warfarin resulted in substantial increases from baseline in the time-to-clot onset, time to 20-mm clot strength, time to maximum amplitude, and decreases in the TEG α-angle, and maximum amplitude (MA) in both the placebo and 80-μg/kg rFVIIa groups (Figure 4). Treatment with 80 μg/kg rFVIIa significantly reversed the effects of warfarin on all TEG parameters compared with placebo, with an increase in the TEG α-angle (P < .001) and maximum amplitude (P = .037) and decreases in time-to-clot onset, time to 20-mm clot strength, and time to maximum amplitude (all P < .001). The effects of rFVIIa on these parameters were sustained up to the last measured time point 5 hours after rFVIIa administration (at B3).
TEG parameters in the placebo or 80-μg/kg groups in experiment 2. TEG parameters in subject treated with placebo or 80 μg/kg rFVIIa at (A) time to clot onset, R, (B) time to achieve 20-mm clot strength, K, (C) angle, (D) maximum amplitude, MA, and (E) time to maximum amplitude, TMA. B0 indicates biopsy 0 (before warfarin treatment); B1, biopsy 1 (after warfarin treatment to achieve an INR of 2.5 ± 0.3); B2, biopsy 2 (15 minutes after rFVIIa or placebo treatment); B3, 5 hours after B2. Arrow indicates time of rFVIIa administration.
TEG parameters in the placebo or 80-μg/kg groups in experiment 2. TEG parameters in subject treated with placebo or 80 μg/kg rFVIIa at (A) time to clot onset, R, (B) time to achieve 20-mm clot strength, K, (C) angle, (D) maximum amplitude, MA, and (E) time to maximum amplitude, TMA. B0 indicates biopsy 0 (before warfarin treatment); B1, biopsy 1 (after warfarin treatment to achieve an INR of 2.5 ± 0.3); B2, biopsy 2 (15 minutes after rFVIIa or placebo treatment); B3, 5 hours after B2. Arrow indicates time of rFVIIa administration.
Safety assessments
No serious adverse events or thromboembolic events were observed. All 68 adverse events were mild or moderate in severity and judged by the investigator as unlikely to be related to rFVIIa administration. A total of 44 subjects experienced at least 1 treatment emergent adverse events: 11 subjects (experiment 1), 2, 5, 5, 4, and 10 subjects (5- to 80-μg/kg rFVIIa groups, respectively) and 7 subjects (placebo) in experiment 2. The most frequently occurring events were headaches (11 events), ecchymosis (6 events), upper respiratory tract infection (5 events), and procedural pain (4 events). Troponin values and mean arterial pressure (based on diastolic and systolic pressure values) remained within the normal range throughout the trial.
Discussion
This exploratory trial was the first known study that concurrently examined the effects of rFVIIa directly on bleeding characteristics and coagulation parameters in healthy human subjects pretreated with warfarin. Warfarin works by depleting vitamin K–dependent coagulation factors and hence prolonging the coagulation process. Warfarin depletion of factors was evident in the current trial from the decreased factor II, FVII, factor IX, and FX levels, prolonged PT and aPTT, and increased INR after warfarin treatment (experiment 1). rFVIIa bypasses the TF-dependent coagulation factors and initiates coagulation by directly activating FX. This was shown in the current trial by the shortened (normalized at higher doses) PTs, aPTTs, and INRs, and normalized coagulation dynamics of TEG and TG. The effect of rFVIIa on aPTT may be the result of activation of FX in the presence of calcium and phospholipid, in the absence of TF,33 and the effect of rFVIIa on PT may indicate that the residual level of FX after warfarin treatment was sufficient for hemostasis to occur.43 Nevertheless, none of the groups showed a complete return to baseline levels in their clotting assays. This was not unexpected, because the multiple coagulation factor deficiencies in the warfarin-treated subjects were only partially compensated for by infusion of rFVIIa. TEG and TG also reflected a sustained normalization effect of rFVIIa, extending up to 2 half-lives of rFVIIa. In contrast, the bleeding duration and blood loss with rFVIIa treatment were not significantly different from placebo.
Despite the common clinical use of PT, INR, and aPTT for evaluating coagulation, these analytes have limited relation to bleeding25,27,28 and depend on the conditions under study.44,45 Further, only the initiation phase of coagulation (involving TF-induced coagulation) is evaluated by the PT assay. TEG and TG assessments, however, provide coagulation profiles that attempt to evaluate initiation, the dynamic development, and final clot strength during clot formation of whole blood.29 Compared with the PT assay, the TG test appears to be more reliable and to more directly reflect bleeding, at least in patients with various coagulation factor deficiencies.26,30,46 When the sensitivity and quality control of the TG assay are optimized with modifications to minimize variability,31,42,47 as in the case of the current trial, then TG may provide a more accurate measure of coagulation status. Technologic advancements have also increased the relevance of TEG in the clinical assessment of bleeding and thrombotic conditions32 and in the monitoring of clot dynamics of both rFVIIa and antagonists to rFVIIa.31 Both assays are thus considered potentially useful in monitoring treatment with rFVIIa.34 The TEG and TG results in this trial may therefore be ex vivo indicators of the potential effect of rFVIIa in warfarin-treated situations. Nevertheless, the incompleteness of the laboratory dataset is a limitation of this study and should be considered in the interpretation of the results.
The effects of rFVIIa on bleeding characteristics (ie, bleeding duration and blood loss) observed in the current trial were not consistent with the ex vivo results from the TEG, TG, PT, aPTT, and INR assays. The variability in absolute baseline bleeding duration and blood loss between experiments 1 and 2 (possibly because of a “practice effect,” because the biopsy was performed manually) makes it difficult to interpret the results of these parameters, although the relative change from baseline was comparable between experiments. Reversal of the effects of warfarin on bleeding time and blood loss has been shown in a previous study in rats by Diness et al43 In that study, bleeding time and blood loss were evaluated from a wound made by a full transection of the tail tip. Administration of 50 μg/kg and 250 μg/kg rFVIIa normalized PT and reduced aPTT in rats pretreated with one dose of warfarin (10 μg/kg). The 250-μg/kg dose completely normalized bleeding in rats given a single dose of warfarin. It is important to note that this was in response to a single warfarin dose.
The evaluation of rFVIIa on bleeding duration and blood loss in the punch biopsy model failed to show a reversal of warfarin-induced coagulopathy, which may reflect the inability of rFVIIa to reverse the effect of warfarin-induced coagulopathy. Other factors may have also influenced this failure, such as the nature of the wound, the distortion of the local anatomy by the administration of local anesthesia used to limit discomfort, the nature of the artificial injury, or other biopsy-related factors such as the location, local vasculature, including the possible transection of even one small blood vessel that could have had a marked effect on bleeding, or other undetermined aspects of the model. Therefore, although variability in the depth of the biopsy was minimized in this study, as inferred from the similarities in biopsy weight determined in a post hoc analysis (data not presented here), the depth of the biopsy may still have been a critical factor affecting the consistency of the bleeding characteristics. Skin bleeding time assessments have been considered to be inconsistent with actual bleeding time in other anatomical parts.48 Studies have shown that platelet aggregation may be unchanged or even increased by warfarin therapy, as a result of the depletion of the physiologic anticoagulants, protein C, and protein S.49,50 In the current study, protein C and protein S were reduced to 35% and 47%, respectively, of their baseline values after warfarin treatment.
The effect of rFVIIa on the warfarin-induced coagulopathy in this in vivo experiment may provide evidence that rFVIIa does not have an effect on this bleeding signal. Note that in the recent review by Rosovsky and Crowther51 that provided a comprehensive review of available evidence, rFVIIa was reported to more rapidly normalize the INR than conventional treatment (eg, FFP + vitamin K), although no comprehensive data were available about cessation of bleeding. Also note that the current result in warfarin-induced coagulopathy stands in contrast to the well-controlled clinical trials in the study of intracerebral hemorrhage,52,53 which repeatedly showed the ability of rFVIIa to limit hemorrhage size (although this was shown in normal coagulation systems) and in the study of patients with penetrating or blunt trauma,54 in which a reduction in blood transfusion requirements was shown with rFVIIa administration.
Therefore, either the lack of effect of rFVIIa or any one or more of these confounding factors could have limited the observable effects of rFVIIa on mitigation of warfarin-induced bleeding in this trial. It appears that the punch biopsy model, although a reproducible model in this setting, may not be the optimal model for studying coagulopathy-induced bleeding. Further, the variability in absolute bleeding duration and blood loss at baseline makes it difficult to interpret the results of this study. Future studies should be designed with these confounding factors in mind.
There were no serious adverse events, thromboembolic events, or any events that resulted in death or withdrawal from this trial. Most of the events were either unlikely to be related to rFVIIa, such as upper respiratory tract infection and headache, or related to the normal side effects of the study procedure, such as excoriation, procedural pain, and catheter site pain. There was only one report of skin infection (cellulitis), which was reported in the 5-μg/kg rFVIIa dose group. It should be noted that the subjects were evaluated for adverse events from time of informed consent until the end-of-trial procedures, so that careful assessments were also being performed during the outpatient portion of the trial when warfarin titration was occurring. Troponin levels remained unchanged and within the normal limits throughout the study.
Conclusion
In this exploratory trial, rFVIIa failed to affect the warfarin-induced changes in bleeding duration and blood loss from the punch biopsy wound. The biopsy bleeding results are inconsistent with the anecdotal reports of bleeding control after rFVIIa use, but these reports are small and typically uncontrolled. In contrast, reversal of the warfarin effect was observed in the TEG, TG, and clotting assays. This reversal did not translate to improvements in the bleeding model parameters, with bleeding durations remaining unchanged in the extended durations after treatment with rFVIIa.
Thus, although it is possible on the one hand that the bleeding model works and the laboratory coagulation assays (which are widely used in many clinical settings) do not accurately reflect in vivo coagulation and therefore that rFVIIa does not have an effect on warfarin-induced bleeding, it is also possible on the other hand that the bleeding model (which is not an established method) does not work, that the laboratory coagulation assays accurately reflect in vivo coagulation, and therefore that rFVIIa does reverse warfarin-induced bleeding. This is a small phase 1 exploratory study on a complex subject with no previous established models. The punch biopsy model was not an established model, unlike other established and widely used bleeding time tests, which are themselves still the subject of controversy about their utility. Therefore, the level of evidence in this study does not bear firm conclusions about the efficacy of rFVIIa in reversing warfarin-induced bleeding in healthy persons.
TG and TEG methods, although useful parameters of in vivo coagulation, failed to reflect any clinical effects on bleeding in this study. Future studies should be designed with these observations in mind.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in abstract form at the 49th annual meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2007.55
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the study volunteers and the facility study staff at Quintiles, Overland Park, KS, and at Novo Nordisk Research Facility US (NNRUS), New Brunswick, NJ, for their contributions to this study. We also thank Don A. Gabriel, MD, PhD, of the University of North Carolina, for his contributions to the safety evaluations for this study.
This work was supported by a grant from Novo Nordisk Inc (D.R.M.).
The sponsor of this study was responsible for providing clinical trial supplies, preparing the study protocol, data management, statistical analysis, and preparing the clinical trial report. N.M.K. and Jessie J. Yun, MSc, are employees of Novo Nordisk and performed the statistical analyses. Charlotte Yap, MSc, a medical writer employed by Novo Nordisk, provided writing and editorial assistance with this manuscript.
Authorship
Contribution: B.E.S. takes responsibility for the integrity of the data and the accuracy of the data analyses; B.E.S., N.M.K., and M.E.C. provided the study concept and design and analyzed and interpreted data; B.E.S., A.E.P., D.R.M., and M.E.C., with the support of the Quintiles study staff, acquired data, supervised the study and provided administrative, technical, or material support; B.E.S. drafted the manuscript; and B.E.S., D.R.M., N.M.K., A.E.P., and M.E.C. critically reviewed and revised the manuscript for important intellectual content. All authors had full access to all the data in the study.
Conflict-of-interest disclosure: Several of the authors (B.E.S., N.M.K., and M.E.C.) are employed by a company or a competitor of a company (Novo Nordisk) whose (potential) product was studied in the present work. The remaining authors declare no competing financial interests.
The current affiliation for A.E.P. is US Army Medical Research and Materiel Command, Fort Detrick, MD.
Correspondence: Brett E. Skolnick, 100 College Rd W, Princeton, NJ 08540; e-mail: bres@novonordisk.com.