Abstract
Th17 cells have never been explored in human graft-versus-host disease (GVHD). We studied the correlation between the presence of Th17 cells with histologic and clinical parameters. We first analyzed a cohort of 40 patients with GVHD of the gastrointestinal tract. Tumor necrosis factor (TNF), TNF receptors, and Fas expression, and apoptotic cells, CD4+IL-17+ cells (Th17), and CD4+Foxp3+ cells (Treg) were quantified. A Th17/Treg ratio less than 1 correlated both with the clinical diagnosis (P < .001) and more than 2 pathologic grades (P < .001). A Th17/Treg ratio less than 1 also correlated with the intensity of apoptosis of epithelial cells (P = .03), Fas expression in the cellular infiltrate (P = .003), TNF, and TNF receptor expression (P < .001). We then assessed Th17/Treg ratio in 2 other independent cohorts; a second cohort of 30 patients and confirmed that Th17/Treg ratio less than 1 correlated with the pathologic grade of GI GVHD. Finally, 15 patients with skin GVHD and 11 patients with skin rash but without pathologic GVHD were studied. Results in this third cohort of patients with skin disease confirmed those found in patients with GI GVHD. These analyses in 96 patients suggest that Th17/Treg ratio could be a sensitive and specific pathologic in situ biomarker of GVHD.
Introduction
Graft-versus-host disease (GVHD) involves dysregulation of inflammatory cytokine cascades and distorted donor cellular response against host alloantigens.1 The characterization of CD4+/interleukin-17 (IL-17)–secreting subset (Th17) and of the regulatory CD4+/Foxp3+ cell (Treg) has had a major impact on our understanding of immune processes.2-6 Th17 and Treg might contribute to human autoimmune diseases, including inflammatory bowel disease.2-5 Experimental data suggest a reciprocal relationship between Th17-induced pathology and Treg-regulatory role. In murine models, Th17 cells induce autoimmunity through tissue inflammation promotion and innate immune system mobilization.2-5 However, in the gut, Th17 cells might also have modulatory and protective roles. Murine models provide recent controversial results on the role of Th17 in GVHD.4,7-11 We are not aware of any published data on Th17 cells in human GVHD. To investigate their potential implication in GVHD-induced inflammation, we studied Th17 and Treg in 3 independent cohorts of patients (n = 96) who had gut or skin biopsies and their relationship with histologic and clinical parameters.
Methods
Patients
Our primary aim was to study Th17 and Treg populations in the setting of gastrointestinal (GI) GVHD. Biopsies were performed as diagnostic procedures for digestive symptoms. Clinical grading used the 1994 consensus conference criteria12 and pathological examinations were performed as previously described.13 The Hôpital Saint-Louis ethical review board approved the design of this study. Patients with digestive symptoms underwent endoscopy and biopsies before any treatment with steroids. We first took advantage of a first study13 in which patient biopsies have been extensively studied for Fas, tumor necrosis factor (TNF), and apoptosis pathways to search for Treg and Th17 infiltration and association with Fas and TNF pathways (if any). Then, we aimed to confirm results of Treg and Th17 localization in an independent cohort of patients with GI GVHD and search for a role (if any) of the intensity of the conditioning regimen. Finally, we studied these cell subsets in patients with skin GVHD. Thus, 3 different cohorts of patients (total n = 96) were sequentially studied:
Forty of 78 patients from the previously published cohort had available duodenal biopsies. Median age was 34 years. Conditioning regimens (all myeloablative) included total body irradiation (TBI) and cyclophosphamide (n = 13), or associated busulfan and cyclophosphamide (n = 15). Acute lymphoblastic leukemia patients received TBI or busulfan with melphalan and cytarabine (n = 9). Aplastic anemia patients received cyclophosphamide and antithymocyte globulins. Cyclosporine and methotrexate were used for most patients (n = 28). Patients underwent duodenal biopsies for unexplained nausea or vomiting (n = 9) or stage 1-2 diarrhea (n = 31).
A second cohort of 30 patients with GI biopsy was studied to confirm in an independent series biologic results in GI GVHD. It included:
Eleven patients (4 males, 7 females; median age, 30 years) with pathologic GI GVHD who underwent an allogeneic stem cell transplantation (SCT) after myeloablative conditioning. Ten patients were grafted for hematologic malignancies. All had stage 2 clinical lower GI GVHD at the time of biopsy.
Ten patients (6 males, 4 females; median age, 48 years) who underwent SCT after a reduced-intensity conditioning that included fludarabine and 2 Gy TBI in 6 patients (according to the Seattle regimen) and low-dose intravenous busulfan fludarabine and antithymocyte globulin in 4 patients (according to the M. D. Anderson regimen). All 10 patients with hematologic malignancies had also biopsy-proven GI GVHD (8 patients had stage 1 and 2 patients had stage 2 lower GI GVHD at the time of biopsy).
Nine patients who had no evidence of pathologic GVHD at the time of upper GI biopsy were used as controls in this second cohort. These patients (6 males, 3 females; median age 30 years) underwent SCT for hematologic malignancies either after a myeloablative (n = 5), or a fludarabine plus 2 Gy TBI reduced intensity conditioning (n = 4; 4 patients had stage 1 lower GI GVHD at the time of biopsy and 5 patients had unexplained nausea/vomiting).
Finally, a third cohort of 26 patients with skin rash was studied. This series included 10 patients with biopsy-proven acute GVHD, 5 patients with evidence of pathologic chronic GVHD, and 11 patients without evidence of GVHD after pathologic examination of skin biopsies. The 10 patients (7 males, 3 females; median age 34 years) with acute GVHD all underwent SCT for hematologic malignancies after a myeloablative conditioning regimen. Four of the 5 patients with chronic GVHD (3 males, 2 females; median age, 43 years) also received a myeloablative conditioning, and all 5 had acute leukemia. Finally, among the 11 patients (7 males, 4 females; median age, 36 years) without pathologic skin GVHD, 9 underwent SCT for malignant diseases and 2 for Fanconi anemia.
Duodenal biopsies during fiberoptic examination and skin biopsies were performed, as previously described.13,14 All biopsies were performed before any steroid treatment, and for GI biopsies 2 of them used for systematic viral or fungal infection detection. Histologic digestive and skin GVHD grading was done as previously described.13
TdT-mediated dUTP nick end labeling (TUNEL) assay and immunohistochemistry were performed on frozen sections using CD45RA (Dako Denmark), CD68 (Dako Denmark), CD95 (BD Biosciences PharMingen), TNF (Genzyme), and TNF receptors, TNFr55 and TNFr75 (IgG monoclonal antibodies, kind gift from M. Brockaus, Switzerland) as primary antibodies. Double immunostainings were performed with Ventana Discovery reagents (Ventana). For Foxp3/CD4 or Foxp3/CD8, incubation with monoclonal mouse antibody against human Foxp3 (clone 22510, Abcam; dilution 1:50), detected by UltraMap detection kit, was followed by incubation with mouse monoclonal antibody against human CD4 (clone 4B12, Ménarini; dilution 1:20) or with monoclonal antibody against human CD8 (clone CD8/144B, Abcam; dilution 1:100) detected by the FastRed detection kit. For IL-17/CD4 double immunostaining, incubation with polyclonal rabbit antibody against IL-17 (H-132sc-7927, Santa Cruz Biotechnology; dilution 1:100), detected by UltraMap detection kit, was followed by an incubation with a mouse monoclonal antibody directed against human CD4 (clone 4B12, Ménarini; dilution 1:20), detected by the FastRed detection kit. Endogenous peroxidase inhibition and nonspecific binding sites blocking were systematically performed. Controls with irrelevant isotypic antibodies and absence of primary antibody were systematically performed. Double immunofluorescent staining was performed on frozen sections for Foxp3/CD4, IL-17/CD4, and Foxp3/IL-17. Primary antibodies were covalently linked to Alexa Fluor 488 or Alexa Fluor 594 using APEX Antibody Labeling Kits (Invitrogen). Sections were incubated in phosphate-buffered saline, pH 7.4, containing 5% bovine serum albumin for 30 minutes at room temperature. Monoclonal mouse antibody to CD4 (clone 4B12, Ménarini; dilution 1:20), monoclonal mouse antibody to Foxp3 (clone 22510, Abcam; dilution 1:50), or polyclonal rabbit antibody to IL-17 (H-132 sc-7927, Santa Cruz Biotechnology; dilution 1:100) were applied to sections for 1 hour at room temperature. Sections were finally mounted in Vectashield medium containing 4,6-diamidino-2-phenylindole. CD4 Th17/CD4 Treg ratio was expressed as median and interquartile range (IQR) of the number of cells per field at ×400 magnification.
Examiners were aware of clinical signs and treatment (if any) of the patients at the time of biopsies and for routine GVHD pathologic evaluation. However, for Th17/Treg staining, patients were identified by anonymous 7-digit codes corresponding to laboratory identification. It is thus unlikely that one examiner could associate patient identification with biopsy code. More importantly, biopsies were independently evaluated by 2 examiners (P.R. and A.J.). In all cases of disagreement between examiners, a common reading was organized to achieve a consensus on count.
Flow cytometry and intracellular cytokine staining
The presence in the peripheral blood mononuclear cells (PBMCs) of CD3+CD4+IL-17+ T cells (Th17) and CD3+CD4+Foxp3+CD25high (Treg) was evaluated by flow cytometry. PBMCs were separated by density gradient centrifugation with lymphocyte separation medium (Organon). After cell surface staining, cells were washed and resuspended in fixation/permeabilization solution (BD, Cytofix/Cytoperm kit; BD Biosciences PharMingen) and intracellular staining was performed following the manufacturer's instructions. For the detection of IL-17, PBMCs were incubated for 4 hours with 50 ng/mL phorbol myristate acetate and 750 ng/mL ionomycin in the presence of monensin (eBioscience) in tissue culture incubator at 37°C.
Conjugated monoclonal antibodies for human CD3 (clone SK7) and CD4 (clone RPA-T4) were purchased from BD Biosciences. Conjugated monoclonal antibodies for human CD25 (clone BC96), Foxp3 (clone PCH101), and IL-17 (clone eBio64DEC17) were purchased from eBioscience. Monoclonal antibodies were conjugated to either fluorescein isothyocyanate, phycoerythrin, phycoerythrin-cyanin 5 (phycoerythrin-Cy5), allophycocyanin, allophycocyanin-7 (allophycocyanin-Cy7), Pacific Blue, and Amcyan.
Stained cells were all analyzed by a BD LSR II flow cytometer, and data were analyzed by FlowJo software (Version 4.1; TreeStar).
Statistical analyses
Reproducibility of Th17 and Treg counts was assessed through examination of discrepancy levels between the 2 examiners and intraclass correlation estimate with 95% confidence interval.15 Th17 or Treg characteristics (cell numbers or their ratio) were summarized through median level and range. Their ability to predict the existence of a clinical or pathologic grade more than 2 GVHD was assessed through the following: sensitivity, proportion of biopsies with a characteristic lower than a given limit in patients with acute clinical or pathologic grade more than 2 GVHD; specificity, proportion of biopsies with a characteristic equal to or higher than the same limit in patients without acute clinical or pathologic grade more than 2 GVHD. The ability of a characteristic to predict clinical or pathologic grade 2 or greater GVHD was tested through 2-sided Fisher exact test, as well as association between the Th17/Treg ratio with other GVHD pathologic markers. For the comparison on proportion of CD4+CD25highFoxp3+ cells, of Th17 cells, and Th17/Treg ratio between the control group and the group with No GVHD on one hand, between the group with No GVHD and the group with acute GVHD on the other hand, Mann-Whitney nonparametric test was used. The same test was used to compare median levels of CD4+ cell counts, proportion of CD4+ cells expressing Foxp3 or IL-17 as a function of pathologic GVHD grade (0-1 vs 2 or more).
Results
Among 100 biopsies evaluated by the 2 examiners (P.R., A.J.), 96 counts showed a difference of 1 (n = 21) or no difference at all (n = 75) for Th17 identified by CD4/IL-17 double staining (Figure 1A-B). For Treg identified by CD4/Foxp3 double staining (Figure 1C-D), the corresponding values were 89, 20, and 69, respectively. Differences were never greater than 2 for Th17 and 3 for Treg (3 biopsies). It should be noted that, for the 11 discrepancies in Treg evaluation, 10 were for counts greater than 5. To summarize the high level of reproducibility between examiners, intraclass correlation was estimated to be 0.950 (95% confidence interval, 0.927-0.966) for Th17 and 0.937 (95% confidence interval, 0.907-0.957) for Treg.
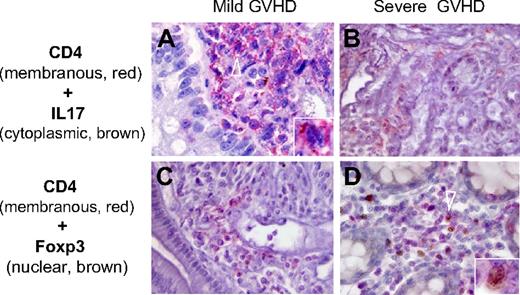
Th17 and Treg distribution in gut biopsies of patients with mild and severe GVHD. Th17 and Treg were identified by the expression of IL-17 or Foxp3, respectively, in CD4+ cells using 2-color immunohistochemistry (A-B, IL-17 in brown; C-D, Foxp3 in brown; A-D, CD4 in red). Th17 cells were more numerous in patients with mild GVHD (A arrowhead), whereas Treg were present in patients with severe GVHD (D arrowhead). Counts of double-immunostained cells were independently assessed by 2 pathologists (P.R. and A.J.) on an Olympus AX 70 microscope with wide-field eyepiece number 26.5. At 400× magnification, this wide-field eyepiece provided a field size of 0.344 mm2.
Th17 and Treg distribution in gut biopsies of patients with mild and severe GVHD. Th17 and Treg were identified by the expression of IL-17 or Foxp3, respectively, in CD4+ cells using 2-color immunohistochemistry (A-B, IL-17 in brown; C-D, Foxp3 in brown; A-D, CD4 in red). Th17 cells were more numerous in patients with mild GVHD (A arrowhead), whereas Treg were present in patients with severe GVHD (D arrowhead). Counts of double-immunostained cells were independently assessed by 2 pathologists (P.R. and A.J.) on an Olympus AX 70 microscope with wide-field eyepiece number 26.5. At 400× magnification, this wide-field eyepiece provided a field size of 0.344 mm2.
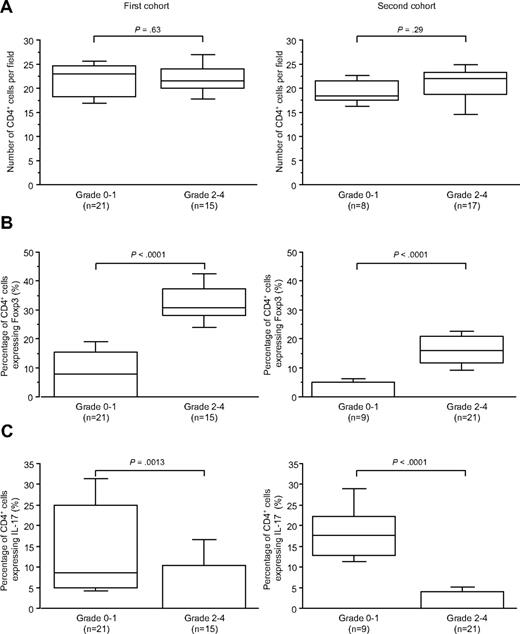
Forty patients with suspected digestive GVHD (nausea/vomiting or diarrhea) had biopsies before any steroid treatment (first cohort); 18 had severe pathologically proven GVHD (pathologic stage > 2; Table 1). Whatever the severity of acute GVHD, the number of CD4+ cells did not vary (P = .63, Figure 2A). As identified by CD4/Foxp3 staining, an increased proportion of CD4+ cells expressing Foxp3 in duodenal biopsies of patients with grade 2 or more compared with patients with grade less than 2 was observed (P < .001, Figure 2B). Conversely, a decreased proportion of CD4+ cells expressing IL-17 identified by and CD4/IL-17 staining in duodenal biopsies of patients with grade 2 or more compared with patients with grade less than 2 was detected (P > .001, Figure 2C).
CD4+counts, CD4+Foxp3+, and CD4+Foxp3+ cell proportions in duodenal biopsies of patients with grade less than 2 or of 2 or more (first and second cohorts). (A) Absolute number of CD4+ cells per field in the first and second cohorts of duodenal biopsies. (B) Percentage of CD4+ cells expressing Foxp3 in the same cohorts. (C) Percentage of CD4+ cells expressing IL-17 in the same cohorts. An increased proportion of CD4+ cells expresses Foxp3 in duodenal biopsies of patients with grade 2 or more compared with patients with grade less than 2 in the 2 cohorts. A decreased proportion of CD4+ cells expresses IL-17 in duodenal biopsies of patients with grade 2 or more compared with patients with grade less than 2 in the 2 cohorts.
CD4+counts, CD4+Foxp3+, and CD4+Foxp3+ cell proportions in duodenal biopsies of patients with grade less than 2 or of 2 or more (first and second cohorts). (A) Absolute number of CD4+ cells per field in the first and second cohorts of duodenal biopsies. (B) Percentage of CD4+ cells expressing Foxp3 in the same cohorts. (C) Percentage of CD4+ cells expressing IL-17 in the same cohorts. An increased proportion of CD4+ cells expresses Foxp3 in duodenal biopsies of patients with grade 2 or more compared with patients with grade less than 2 in the 2 cohorts. A decreased proportion of CD4+ cells expresses IL-17 in duodenal biopsies of patients with grade 2 or more compared with patients with grade less than 2 in the 2 cohorts.
The numbers of CD4+ expressing IL-17 or Foxp3 was low (Figure 1): CD4 Th17 ranging from 0 to 8 cells per field with a median of 1 (IQR = 0.25-4); CD4 Treg median being 4 (IQR = 1-6), with a median Th17/Treg ratio of ½ (Table 1). Severe apoptosis, assessed by TUNEL, was found in 29 patients, Fas+ mononuclear cells in 23, significant TNF-α expression in 20 and TNF receptor 1 in 26 (Table 2).
In the 34 patients with pathologic GVHD, CD4 Th17 cells numbers ranged between 0 and 7 with a median of 1, whereas CD4-Treg ranged between 0 and 11 with a median of 5 (Table 1). A Th17/Treg ratio less than 1 correlated both with the clinical (sensitivity 74%, specificity 100%, P = .001) and pathologic grade more than 2 GVHD (sensitivity 94%, specificity 64%, P < .001). Apoptotic epithelial cells were associated with more than 4 Th17 cells per field in only 3 biopsies but with more than 4 Treg cells in 19 of 34 biopsies. In the 6 biopsies without apoptotic cells, the corresponding values were 5 and 0, respectively. A similar trend was found for Fas+ mononuclear cells. In the 17 biopsies with Fas− cells, the corresponding values were 7 and 1. In 20 biopsies with high TNF-α expression, none had more than 4 Th17 cells, whereas 17 had more than 4 Treg cells per field. In 20 biopsies without high TNF expression, the corresponding values were 8 and 2. As a consequence, a Th17/Treg ratio less than 1 correlated with the intensity of apoptosis of epithelial cells (quantitative TUNEL assay), with Fas expression in the cellular infiltrate, and interestingly highly correlated with TNF and TNF receptor 1 and 2 expression (P < .001 for all; Table 2).
We next wanted to confirm, in an independent cohort (second cohort), results concerning Th17 and Treg cells in human GI GVHD and to examine whether the intensity of the conditioning regimen (reduced intensity vs myeloablative) influences the expression ratio of these 2 cell subsets. As summarized in Figure 2 and Table 3, we found nearly the same results as in the previously studied cohort regarding Th17 and Treg cell density with regard to GVHD occurrence. However, neither Th17 (P = .9) or Treg cells (P = .5) numbers, nor Th17/Treg were influenced by the type of conditioning regimen.
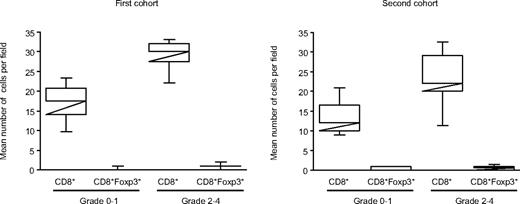
Although Foxp3 has a central role in Treg development, it is also clear that Foxp3 up-regulation occurs with T-cell activation. Thus, as an additional control of the specificity of our results, we used CD8/Foxp3 double staining on the 2 first cohorts. As shown in Figure 3, CD8+Foxp3+ cell counts were negligible compared with CD8+ cells in both first and second cohort whatever the GVHD status (median of 1 and 0.5 cells per field for CD8+Foxp3+, and 30 and 25.5 cells for CD8+, respectively; Figure 3).
CD8+ and CD8+Foxp3+ cells in duodenal biopsies in the first and second cohorts. Number of CD8+ and CD8+Foxp3+ cells in GVHD patients with grade less than 2 or 2 or more. Although Foxp3 has a central role in Treg development, it is also clear that Foxp3 up-regulation occurs with T-cell activation. Thus, as a control of the specificity, CD8/Foxp3 double staining was performed on the 2 first cohorts. Counts showed that most of the CD8+ cells are not activated in the 2 independent cohorts of patients who underwent duodenal biopsies.
CD8+ and CD8+Foxp3+ cells in duodenal biopsies in the first and second cohorts. Number of CD8+ and CD8+Foxp3+ cells in GVHD patients with grade less than 2 or 2 or more. Although Foxp3 has a central role in Treg development, it is also clear that Foxp3 up-regulation occurs with T-cell activation. Thus, as a control of the specificity, CD8/Foxp3 double staining was performed on the 2 first cohorts. Counts showed that most of the CD8+ cells are not activated in the 2 independent cohorts of patients who underwent duodenal biopsies.
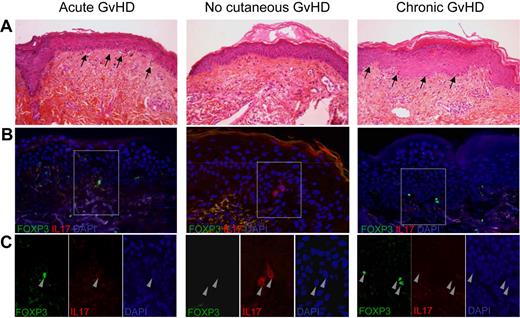
Because recent experimental results in a mouse model suggest that Th17 cells might be more involved in skin GVHD rather than in GI GVHD,4,7-11 we analyzed data in an additional cohort of 25 patients (third cohort) with acute skin rash or chronic lichenoid skin lesions who underwent skin biopsies (Figure 4). As summarized in Table 4, we found again similar results regarding Th17 and Treg cell ratio with regard to acute GVHD occurrence. Results in chronic GVHD were statistically insignificant with regard to Th17 and Treg cell numbers but showed similar trends with regards to Th17/Treg.
Double immunofluorescent staining with IL-17 and Foxp3 in skin biopsies of patients with acute, chronic, and no GVHD. (A) Skin biopsies showed infiltrates and apoptotic bodies (arrows) in acute and chronic lichenoid eruption but not in patients without GVHD. (B) Overlay of double immunofluorescent staining with IL-17 (red) and Foxp3 (green) antibodies showed Foxp3 expressing cells in skin biopsies of patients with acute and chronic GVHD and IL17-expressing cells in skin biopsies of patients without GVHD. (C) Enlargement of the stained cells showed that none of them expressed both IL-17 and Foxp3.
Double immunofluorescent staining with IL-17 and Foxp3 in skin biopsies of patients with acute, chronic, and no GVHD. (A) Skin biopsies showed infiltrates and apoptotic bodies (arrows) in acute and chronic lichenoid eruption but not in patients without GVHD. (B) Overlay of double immunofluorescent staining with IL-17 (red) and Foxp3 (green) antibodies showed Foxp3 expressing cells in skin biopsies of patients with acute and chronic GVHD and IL17-expressing cells in skin biopsies of patients without GVHD. (C) Enlargement of the stained cells showed that none of them expressed both IL-17 and Foxp3.
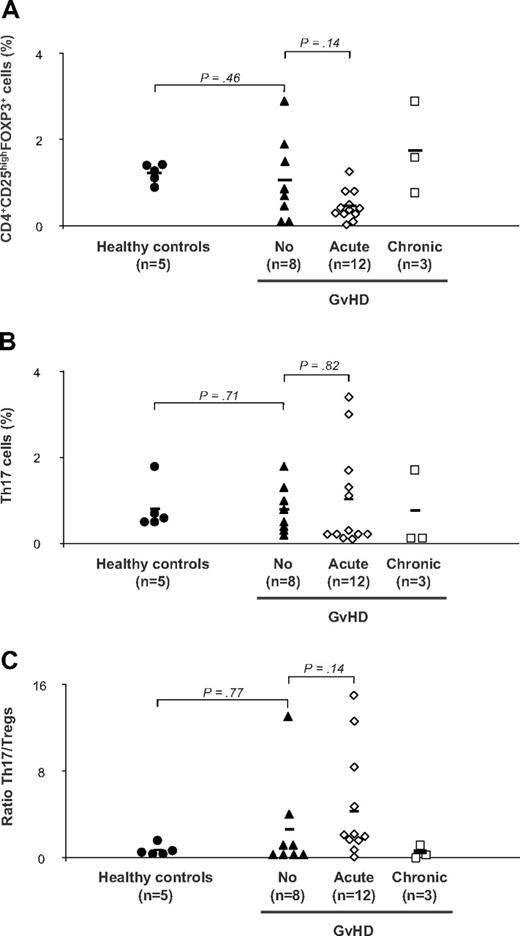
Finally, we assessed the presence of Th17 and Treg subsets in the PBMCs of 31 patients of the second (GI biopsied) and third cohort (skin biopsied). Samples were analyzed from prospectively collected material at a mean of 3 months after transplantation. Five healthy persons were included as controls. Because some patients were highly lymphopenic after hematopoietic stem cell transplantation, both subsets were detectable in 23 of the 31 patients. Th17 and Treg frequencies were then correlated to the presence of GVHD. Within the 23 patients, 8 did not have sign of GVHD at time of sampling, whereas 12 presented acute and 3 chronic GVHD. The Treg cell percentage was significantly lower in the 12 patients with acute GVHD than in the 8 patients without GVHD (1.6% ± 0.35% vs 0.46% ± 0.1%; P < .001; Figure 5A). For the 3 patients with chronic GVHD, the percentage of Treg cells was 1.7% plus or minus 1.0%. However, we found no correlation between Th17 cell percentage and GVHD occurrence (Figure 5B). The Th17/Treg ratio was statistically no different between patients with or without signs of acute GVHD (P = .14, Figure 5C).
Flow cytometry analyses of Th17 and of Tregs in the peripheral blood of 31 patients with acute, chronic, or no chronic GVHD. Flow cytometry analyses of Th17 and of Tregs in the peripheral blood of 23 patients with acute, chronic, or no chronic GVHD. PBMCs from healthy controls (n = 5) and patients with GVHD (acute, n = 12 and chronic, n = 3) or without (n = 8) were stained with anti-CD3, anti-CD4, anti-CD25 antibodies followed by intracellular Foxp3 antibody and examined by flow cytometry. Intracellular IL-17 antibody was also examined by flow cytometry after stimulation for 6 hours with phorbol myristate acetate and ionomycin. (A) Frequencies of Tregs in patients and controls. (B) Frequencies of Th17 T cells in patients and controls. (C) Th17/Tregs ratio in patients and controls. The mean value of each group is represented (solid line).
Flow cytometry analyses of Th17 and of Tregs in the peripheral blood of 31 patients with acute, chronic, or no chronic GVHD. Flow cytometry analyses of Th17 and of Tregs in the peripheral blood of 23 patients with acute, chronic, or no chronic GVHD. PBMCs from healthy controls (n = 5) and patients with GVHD (acute, n = 12 and chronic, n = 3) or without (n = 8) were stained with anti-CD3, anti-CD4, anti-CD25 antibodies followed by intracellular Foxp3 antibody and examined by flow cytometry. Intracellular IL-17 antibody was also examined by flow cytometry after stimulation for 6 hours with phorbol myristate acetate and ionomycin. (A) Frequencies of Tregs in patients and controls. (B) Frequencies of Th17 T cells in patients and controls. (C) Th17/Tregs ratio in patients and controls. The mean value of each group is represented (solid line).
Discussion
In total, 96 patient biopsies have been analyzed to assess the potential role of Th17 cells in human GVHD. We first explored a cohort of 40 patients with suspected GVHD of the GI tract. Th17/Treg correlated both with the clinical diagnosis and disease severity as assessed by pathologic grade or by the intensity of the alloimmune reaction (apoptosis of epithelial cells, Fas expression, TNF and TNF receptors). We then assessed Th17/Treg ratio in 2 other independent cohorts; a second cohort of patients with GI biopsies confirmed that Th17/Treg ratio less than 1 correlated both with the clinical diagnosis and pathologic grade irrespective of what the pretransplantation conditioning regimen was. Finally, patients with skin GVHD were studied, and results in this last cohort of patients with skin disease confirmed those found in patients with GI GVHD.
The Th17/Treg ratio we found in this human GVHD study does not support the hypothesis of a reciprocal relationship between Th17-induced pathology and Treg regulatory role proposed for autoimmune diseases,2,16 especially in the GI tract. CD4 Th17 cells have been characterized in situ in human autoimmune diseases, including Sjogren syndrome, Crohn disease, and rheumatoid arthritis in humans.17-20 In this first study of Th17 in human GVHD, we found a lower number of CD4 Th17 cells than in our 5 controls of inflammatory active Crohn disease. This lower percentage of Th17 in GVHD compared with Crohn disease should be kept in mind because this latter disease is usually considered as the prototypic Th1-Th17 GI disease.21 Treg cells numbers we detected in the GVHD biopsies are in agreement with Rieger et al22 who found a mean of 5.4 cells/field and few Treg cells around crypts in acute GVHD patients. It is of note, however, that, contrary to these authors, we did not found increased numbers of Treg in patients without GVHD.
Today all our knowledge on the role of Th17 cells in GVHD comes from experimental mice studies that lead to divergent conclusions. The role of Th17 has been studied in idiopathic pneumonia syndrome and chronic GVHD rodent models.8,9 Th17 was most recently studied in experimental acute GVHD. Carlson et al7 reported that a high dose of in vitro differentiated Th17 mediated severe cutaneous and pulmonary lesions. Yi et al11 showed that IL-17−/− donor T cells had high Th1 differentiation and induced severe acute GVHD damage. This is in accordance with the results of our human study, where a low Th17/Treg ratio also correlated with severe clinical and pathologic GVHD, apoptosis intensity, and TNF-α expression. However, a limitation inherent to our human study design is that no sequential biopsies (ie, in nonsymptomatic patients early after transplantation) were performed. Thus, recent experimental results of Kappel et al4 and Iclozan et al,23 suggesting that Th17 contributes to the early phase of CD4-mediated GVHD, cannot be ascertained in the present study. In another recent study, Yi et al10 used either wild-type mice donor CD4+ T cells that lead to a predominantly Th1 cell–mediated disease that preferentially mediated GVHD tissue damage in the gut and liver. However, when authors used interferon-γ−/− CD4+ T cells, GVHD that enhanced Th2 and Th17 differentiation and exacerbated tissue damage in the lung and skin; the absence of both IL-4 and interferon-γ resulted in enhanced Th17 differentiation and preferential, although not exclusive, tissue damage in the skin. The tissue-specific GVHD mediated by Th1, Th2, and Th17 cells was, in part, associated with their tissue-specific migration mediated by differential expression of chemokine receptors. In view of such results and those obtained using Th17-expanded cells that also induced a skin prominent disease, we also analyzed skin biopsies, but we did not find evidence for Th17 cells expansion in skin GVHD in humans. It should also be stressed that cyclosporine A used in human to prevent GVHD also inhibits IL-17 secretion24 and may have altered fate of Th17 cells in human GVHD.
Most recently, the relationship between Th17 and Treg has gained a further level of complexity. Indeed, CD25highFoxp3+ regulatory T cells might be able to differentiate into IL-17–producing cells.25,26 This has also to be considered for GVHD analysis in human. However, we never found cells coexpressing IL-17 and Foxp3 in human biopsies.
Although this study represents the first one in human GVHD and is based on the analysis of nearly 100 biopsies, we acknowledge some limitations. First, a larger number of patients with chronic GVHD should be studied. Second, a biopsy is only able to depict a situation (photography) at a given time after transplantation, even if this time corresponds to early onset of clinical signs. Analyses we performed on these 2 cell subsets in peripheral blood found no correlation between Th17 cell percentage and GVHD occurrence, and the Th17/Treg ratio was not statistically different between patients with or without signs of GVHD. Thus, with the limitation inherent to human studies, we cannot rule out that early pathogenetic mechanisms in human GVHD involve a prominent role of the Th17 cell subset. This is particularly relevant in view of recent results by Litjens et al27 who demonstrated in vitro that the repertoire of alloreactive CD4+ T cells is biased to a Th17 response with an average 24% of alloreactive CD154+CD4+ memory T cells producing IL-17 after polyclonal stimulation. Unexpectedly, in this study, mixed cell cultures from human leukocyte antigen–identical donors also generated alloreactive CD154+CD4+ T cells and yielded the highest frequency compared with human leukocyte antigen–nonidentical combinations.
In conclusion, Th17 in human GVHD were not associated with evidence of severe tissue damage at disease onset, but in situ quantification of the Th17/Treg ratio was a specific marker of human GVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Stéphanie Belhadj for excellent technical assistance.
This work was supported by the European Commission Stem-Diagnositics (contract no. LSHB-CT-2007-037703), Institut National du Cancer and Cancéropôle Ile de France, Conseil régional Ile de France, and Agence de Biomédecine. R.P.d.L. received a bursary award from the Aplastic Anemia and Myelodysplastic Syndrome International Foundation.
Authorship
Contribution: G.S. designed the study and, with P.R. and A.J., analyzed the data and wrote the manuscript; C.L. and A.D. performed immunochemistry analyses; P.B. contributed to immunohistochemistry analyses; E.C. and C.D. collected and prepared PBMCs; K.K. and R.P.d.L. performed flow cytometry and analyzed data; C.P. and A.Q. collected and summarized clinical data; M.R., P.R., and R.P.d.L. recruited patients and participated in manuscript discussion; and J.Y.M. performed statistical analyses and actively participated in the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gérard Socié, Service d'Hématologie Greffe, Inserm U728, Hôpital Saint-Louis, 1 Av Vellefaux, 75010 Paris, France; e-mail: gerard.socie@sls.aphp.fr.
References
Author notes
P.R. and A.J. contributed equally to this study and should be considered as sharing first authorship.
R.P.d.L. and C.L. contributed equally to this study and should be considered as sharing equal authorship.