In this issue of Blood, Ağar and colleagues present data for a novel explanation of how an antigenic target on β2GPI, a central protein in the APS disease process, can become available for binding by antibodies.1
The antiphospholipid syndrome (APS), an autoimmune thrombophilic disorder, was recognized as a diagnostic entity by astute clinical observations of the coincidence of thrombosis and/or recurrent miscarriages with empirically derived clinical tests.2 These include immunoassays that were derived from the biologic false-positive syphilis test and blood tests that detect inhibitors of phospholipid-dependent coagulation reactions, known as lupus anticoagulant assays.
In retrospect, the “antiphospholipid” terminology is erroneous and reflects the initial belief that phospholipids themselves are the targets of the antibodies. This misconception—but not the name of the syndrome—was corrected approximately 20 years ago, when it was discovered that the actual target antigens are phospholipid-binding proteins, particularly β2-glycoprotein I (β2GPI), a relatively abundant plasma protein whose biologic function(s) has not been established.
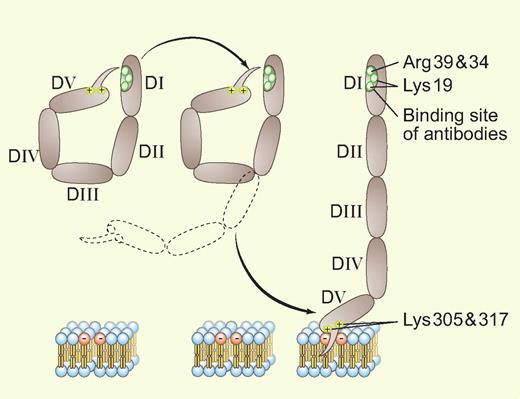
X-ray crystallographic studies3,4 revealed that the protein, with its 5 homologous domains, has a J-shaped structure that is analogous to a fishhook with a “barb” consisting of a hydrophobic loop with surrounding positively charged residues near the carboxyterminus on domain V. This region allows the protein to bind bilayers containing anionic phospholipids via affinity for negatively charged polar heads and insertion of the loop within the hydrophobic middle of the bilayer (see figure).
An adaptation of Ağar et al's Figure 71 showing the transition between the 2 conformations of β2GPI. Professional illustration by Paulette Dennis.
An adaptation of Ağar et al's Figure 71 showing the transition between the 2 conformations of β2GPI. Professional illustration by Paulette Dennis.
While several investigators have reported different specificities for the antibodies, there is significant evidence that patients having antibodies recognizing an epitope in domain I are at an increased risk for thrombosis.5 It has been proposed that the antibodies may dimerize or perhaps multimerize the protein and that the multivalency of these antibody-β2GPI complexes for phospholipids would increase their affinity/avidity for the bilayers (and potentially other receptors), which in turn would amplify the putative downstream thrombogenic effects of the antibodies. The latter might occur via disruption of endogenous anticoagulant mechanisms on vascular endothelial cells or by triggering signaling events that induce prothrombotic programs (eg, expression of tissue factor or cell-adhesion molecules).
Ağar et al address the specific question of why these antibodies can only recognize domain I after the β2GPI has become bound to phospholipid6 but not unbound β2GPI in solution. The specifics of this process were not previously understood, but were presumed caused by conformational change(s). Ağar et al provide convincing evidence for a simple and elegant mechanism: unbound β2GPI present in solution exists in a closed circular conformation—in effect, as a coiled fishhook—in which domain V is noncovalently bound to domain I and thereby shields the domain I epitope from availability for antibody recognition. The binding of β2GPI to a phospholipid bilayer via the “barb” on domain V unsnaps this coiled protein into its open fishhook conformation, thereby exposing the epitope (see figure). The authors present a convincing body of evidence for this idea, including electron microscopic images, differential trypsin digestion profiles, surface plasmon resonance binding studies of the affinity of recombinant domains for each other, and functional studies that compare the anticoagulant effects of the conformations.
These results add a significant detail in our understanding of the APS disease process, which can be outlined as follows: β2GPI is present in plasma where it has been suggested to play a role in the clearance of apoptotic cells and microparticles. The circular protein then undergoes a conformational change when it comes into contact with membranes of cells that have entered the apoptotic program and express anionic phospholipids; domain V unsnaps from domain I and inserts into the bilayer and the fishhooks agglomerate into disc-like clusters,7,8 a process that is probably required for the protein's biologic role. In patients who have a genetic susceptibility for developing APS, the exposure of the neoepitope on domain I may trigger an autoimmune response and stimulate formation of aPL antibody-β2GPI immune complexes on the surface of the cell membranes.8 In turn, these antiphospholipid antibody-β2GPI immune complexes may exert a variety of effects on the cell membranes, including interference with membrane-mediated antithrombotic mechanisms such as A5 crystallization, protein C activation, and annexin A2–mediated fibrinolysis, as well as stimulation of a prothrombotic and proadhesive phenotype, activation of complement, and perhaps effects on the rigidity of the cell membranes.
There were a few limitations in this study. Having the direct evidence of EM images of β2GPI bound to phospholipid would have been helpful; however, the authors report that this was not technically feasible because the protein aggregated lipid vesicles. In addition, the 2 conformations of purified β2GPI were prepared by dialyzing the purified protein against nonphysiologic buffers: alkaline pH and a high salt concentration for the open conformation and an acidic buffer for the closed conformation. Finally, IgG fractions from only a small group of 3 patients were used to show that the antibodies recognize the open, but not the closed, conformation of β2GPI; it would be interesting to know whether analysis of a larger number of APS patients would confirm this to be a consistent finding or show evidence for heterogeneity.
Nevertheless, this work is an important contribution that advances the understanding of an early step in the APS disease process and may provide new insights for improved diagnosis and treatment of this disorder. Learning whether this “coiled fishhook” model for conformational change is found to occur with other binding proteins will also be interesting.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■