Plasma cell dyscrasias are frequently encountered malignancies often associated with kidney disease through the production of monoclonal immunoglobulin (Ig). Paraproteins can cause a remarkably diverse set of pathologic patterns in the kidney and recent progress has been made in explaining the molecular mechanisms of paraprotein-mediated kidney injury. Other recent advances in the field include the introduction of an assay for free light chains and the use of novel antiplasma cell agents that can reverse renal failure in some cases. The role of stem cell transplantation, plasma exchange, and kidney transplantation in the management of patients with paraprotein-related kidney disease continues to evolve.
Introduction
Monoclonal plasma cell disorders are common with monoclonal gammopathy of undetermined significance (MGUS) affecting up to 3.2% of all patients over the age of 50 and with multiple myeloma (MM) accounting for 10% of all hematologic malignancies.1,2 Renal insufficiency, defined by abnormal creatinine clearance, is present in up to half of myeloma patients at presentation, contributes to excessive early mortality, and diminishes eligibility and clinical outcomes after both systemic therapy and high-dose stem cell transplantation (SCT), as well as novel treatments.3,4 Indeed, reversibility of myeloma-associated renal impairment is a critically important prognostic factor and even supercedes response to systemic therapy as a predictor of improved survival.5 This review will discuss the mechanisms of kidney injury in monoclonal plasma cell disorders, while highlighting recent advances in the detection of monoclonal immunoglobulin (Ig), the availability of new renoprotective chemotherapeutic approaches, and the ongoing research into the roles of plasmapheresis and kidney transplantation in these diseases.
Monoclonal plasma cell disorders are a spectrum of diseases that includes premalignant MGUS, solitary plasmacytoma, Ig-mediated amyloidosis (AL amyloidosis), and both asymptomatic and symptomatic MM.6 Plasma cell clones across the entire spectrum exhibit malignant features including abnormal cell surface protein expression patterns and typically proliferate slowly, with a growth fraction of usually less than 1%.7 Virtually all myeloma cells and most clonal plasma cells associated with MGUS also have chromosomal abnormalities consisting of gene rearrangements or hyper- or hypodiploidy, which contribute to dysregulation of intracellular signaling pathways and in some cases are associated with progressive disease and a poor prognosis.8 The molecular events underlying the progression of MGUS to MM remain uncertain but may result from alterations in antitumor immunity as well as changes in the tumor-related microenvironment.9 As a paradigm of an emerging theme in myeloma as well as other areas of cancer biology, interactions among malignant plasma cells, bone marrow stromal cells, and extracellular matrix are now known to be critical to myeloma proliferation and chemotherapeutic resistance.10
Clinical presentation
Whether kidney impairment is present or not, the initial symptoms of myeloma and related plasma cell dyscrasias can be insidious and include fatigue, weight loss, and bone pain. Although most patients are older than 60 years of age, as many as 10% of myeloma patients are younger than 40 years of age (with, in 1 series, the youngest patient reported being 19 years old).11 Anemia, which is a traditional hallmark of myeloma, is present in up to 75% of patients at presentation. Patients with renal end-organ dysfunction and monoclonal Ig deposition disease (MIDD) are often hypertensive, whereas patients with amyloid may be hypotensive or orthostatic. Patients may also present with symptoms from systemic amyloid or monoclonal Ig deposition, including congestive heart failure, cardiac arrthymias, hepatomegaly, portal hypertension, and/or periorbital purpura.12 Conversely, gastrointestinal bleeding or perforation and elevated alkaline phosphatase are highly suggestive of amyloidosis affecting the gastrointestinal tract and liver, respectively.
The urine sediment is typically bland in patients with myeloma though a minority of patients with MIDD (∼ 20%) will have microscopic hematuria.12 In rare patients with monoclonal Ig-mediated glomerulonephritis, the urine sediment is active with red and white cells and cellular casts. Urine dipsticks are insensitive to cationic proteins like Ig, but tests of total urine protein are often abnormal. A simultaneously high urine total protein and low microalbumin (both normalized for urine creatinine) is highly suggestive of light chain proteinuria and should be confirmed with specific techniques to detect monoclonal Ig, as described below (see “Detection of monoclonal Ig”). Nephrotic range albuminuria suggests amyloidosis or glomerular deposition of monoclonal Ig. Myeloma may be present even when the urine is free from albumin, and so specific tests for monoclonal Ig should always be pursued if the index of suspicion is high.
Additional laboratory clues that might prompt testing for monoclonal Ig include a high or low serum globulin fraction, an abnormally low anion gap, unexplained hyponatremia, hypercalcemia, hypophosphatemia, or hyperphosphatemia, the Fanconi syndrome, distal renal tubular acidosis, a low high-density lipoprotein cholesterol, or nephrogenic diabetes insipidus.13 Complement levels are normal but may be low in cases of heavy chain or IgM deposition.14 Kidney imaging such as ultrasonography can be helpful in excluding obstruction but is of limited value in the diagnosis of monoclonal Ig-associated kidney failure, although patients with amyloid or plasma cell infiltration may have enlarged kidneys.
Myeloma is diagnosed when a serum monoclonal protein component is present (typically in excess of 3 g/dL), the fraction of bone marrow plasma cells is greater than 10% and there is evidence of end-organ injury, such as hypercalcemia, renal dysfunction, anemia, or bone disease. Patients with kidney damage from Ig deposition who have clonal plasma cells in their bone marrow are characterized as having symptomatic myeloma regardless of the size of the monoclonal protein or the percentage of bone marrow plasma cells.6
Many patients present with mild chronic kidney disease (CKD) and low levels of monoclonal Ig of unclear significance. In these cases a bone marrow aspirate and biopsy will sometimes show plasmacytosis consistent with myeloma and so provide sufficient grounds for treatment. Conventional cytogenetics, fluorescence in situ hybridization, and flow cytometry add additional prognostic information.6 If uncertainty remains, persistent or rising levels of monoclonal Ig combined with unexplained or worsening CKD may warrant kidney biopsy to document Ig deposition and provide a rationale for antiplasma cell therapy.
Mechanisms of kidney injury in plasma cell malignancies
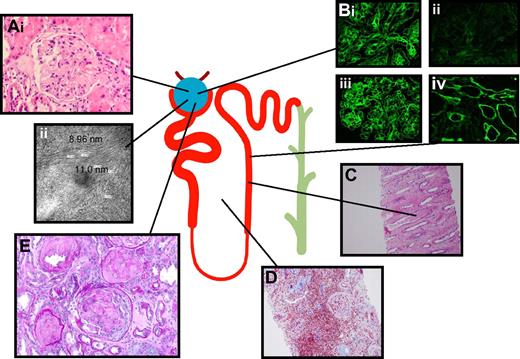
Mechanisms of kidney injury in plasma cell malignancies can be broadly grouped into Ig-dependent and -independent categories, as shown in Table 1, though in particular patients several mechanisms often coexist and interact to produce kidney injury. As illustrated in Figure 1, the 3 most common forms of Ig-dependent kidney injury include: (1) cast nephropathy, in which casts and crystals composed of filtered monoclonal Ig and other urinary proteins obstruct distal renal tubules, often precipitously, and typically incite an accompanying tubulointerstitial nephritis; (2) AL amyloidosis, in which primarily monoclonal light chains and other proteins together form β-pleated sheets in the glomeruli; or (3) MIDD, in which intact or fragmented light chains, heavy chains, or both deposit along glomerular and/or tubular basement membranes.
Three distinct syndromes account for most cases of Ig-mediated kidney disease but virtually all nephropathologic syndromes have been observed. Panel A shows amyloid. (i) Amyloid fibrils consisting of monoclonal Ig and serum proteins appear here as pink material disrupting glomeruli architecture. (ii) Amyloid is visible on electron microscopy as 7- to 12-nm fibrils. Panel B shows MIDD. Monoclonal light chains kappa (1) or without evidence of lambda (ii) and/or heavy chains (IgG), deposit along glomerular (iii) and tubular basement membranes (iv), altering the glomerular structure and causing dose-dependent proximal tubular toxicity. Panel C shows cast nephropathy. Filtered monoclonal Ig, Tamm-Horsfall, and other proteins form casts, which obstruct tubules and collecting ducts. Casts can rupture and result in interstitial inflammation. Panel D shows interstitial inflammation. Inflammation also results from the processing of filtered monoclonal light chains, which induces NF-κB and other signaling pathways leading to cytokine-mediated inflammatory infiltrate (shown here with a Trichrome stain) and subsequent matrix deposition and fibrosis. Panel E shows glomerular crescent. Virtually every recognized nephropathologic lesion has been described in association with paraproteinemia. Shown here is a glomerular crescent in a patient with Waldenström macroglobulinemia productive of IgMλ and amyloidosis.
Three distinct syndromes account for most cases of Ig-mediated kidney disease but virtually all nephropathologic syndromes have been observed. Panel A shows amyloid. (i) Amyloid fibrils consisting of monoclonal Ig and serum proteins appear here as pink material disrupting glomeruli architecture. (ii) Amyloid is visible on electron microscopy as 7- to 12-nm fibrils. Panel B shows MIDD. Monoclonal light chains kappa (1) or without evidence of lambda (ii) and/or heavy chains (IgG), deposit along glomerular (iii) and tubular basement membranes (iv), altering the glomerular structure and causing dose-dependent proximal tubular toxicity. Panel C shows cast nephropathy. Filtered monoclonal Ig, Tamm-Horsfall, and other proteins form casts, which obstruct tubules and collecting ducts. Casts can rupture and result in interstitial inflammation. Panel D shows interstitial inflammation. Inflammation also results from the processing of filtered monoclonal light chains, which induces NF-κB and other signaling pathways leading to cytokine-mediated inflammatory infiltrate (shown here with a Trichrome stain) and subsequent matrix deposition and fibrosis. Panel E shows glomerular crescent. Virtually every recognized nephropathologic lesion has been described in association with paraproteinemia. Shown here is a glomerular crescent in a patient with Waldenström macroglobulinemia productive of IgMλ and amyloidosis.
It is important to note that the complete spectrum of renal disease associated with monoclonal Ig can be much broader than the 3 syndromes described in the paragraph above and includes nearly every renal pathologic entity. Glomerulonephritis may occur, of either a membranoproliferative, diffuse proliferative, crescentic, or cryoglobulinemic variety.15 Nephrotic syndrome may result from an Ig-induced minimal change or membranous lesion.16,17 Renal limited thrombotic microangiopathy can result directly from monoclonal Ig-induced endothelial injury or after chemotherapy or SCT.18 IgA myeloma can rarely result in Henoch-Schonlein purpura and IgA nephropathy.19 Patients with circulating monoclonal IgM can develop kidney injury from hyperviscosity or when deposits composed of monoclonal IgM occlude glomerular capillaries.20 Crystalline inclusions in the proximal tubule consisting of monoclonal Ig can result in a mild tubulointerstitial nephritis or the Fanconi syndrome, which is often indolent and may not require systemic therapy.21,–23
Most myelomas secrete intact Ig with relatively small amounts of accompanying monoclonal light chain. However, myelomas that only secrete light chains account for approximately 20% of myeloma cases and cause 40% to 60% of severe acute kidney injury associated with the disease.6 Unsurprisingly, nonsecretory myeloma rarely causes kidney injury. Clinical experience teaches that renal dysfunction is inevitable in most patients over the course of the natural history of myeloma. Patients at particularly high risk are those with more than 10 g/day of light chain excretion and those with IgD myeloma, 70% and 100% of whom, respectively, will develop kidney injury.4 On occasion patients who do not meet the criteria for any of the monoclonal plasma cell disorders will develop MIDD as a result of a small, difficult to detect plasma cell clone producing a nephrotoxic monoclonal Ig.24
Several Ig-independent mechanisms commonly cause or contribute to kidney injury in plasma cell malignancy, including volume depletion, sepsis, pyelonephritis, hypercalcemia, uric acid nephropathy, rhabdomyolysis, direct renal parenchymal invasion by plasma cells, nonsteroidal anti-inflammatory drugs, and renin-angiotensin system inhibitors (Table 1).25 Patients with myeloma receiving the amino-bisphosphonate zoledronate have developed acute kidney injury, whereas others treated with its counterpart pamidronate have developed nephrotic syndrome from collapsing focal segmental glomerulosclerosis.26 In an autopsy series of 77 myeloma patients, acute tubular necrosis (possibly related to nephrotoxic light chains) was the single most common kidney lesion, followed by cast nephropathy, with several patients demonstrating MIDD or AL amyloidosis, which was unsuspected before death.18
Kidney biopsy should be considered early in the course of renal impairment, when the serum creatinine has risen no more than 25% to 30%, to provide a definitive diagnosis and distinguish between Ig- and non-Ig–mediated mechanisms. Special techniques such as pronase digestion and immunogold labeling may be required to detect and characterize monoclonal Ig and amyloid.27 In cast nephropathy, the dominant finding is tubular casts in the distal nephron, often with accompanying interstitial nephritis.18 In MIDD, tubular and glomerular basement membranes are thickened by refractile precipitates that are granular and dense on electron microscopy.28 Glomerular nodules resembling the Kimmelstiel-Wilson lesion of diabetic nephropathy may be present and cause the nephrotic syndrome. In AL amyloidosis, the tubular basement membranes are typically of normal thickness but the Congo red stain is positive, with characteristic green birefringence under polarizing microscopy. Electron microscopy demonstrates organized deposits of nonbranching amyloid fibrils, 7 to 10 nm in diameter.
Monoclonal Igs injure renal tissue through a diverse set of mechanisms. After filtration and clathrin-dependent endocytosis via the cubilin/megalin scavenger receptor system, light chains may resist degradation, precipitate, and cause proximal tubule dysfunction.29,30 Processing of pathologically large quantities of monoclonal light chains by proximal tubular cells may result in the production of proinflammatory cytokines such as interleukin-6 and interleukin-8, and monocyte chemoattractant protein-1.31,32 Light chain endocytosis and processing results in epithelial-mesenchymal transformation of tubular epithelial cells and catalyzes the production of hydrogen peroxide and other reactive oxygen species, further contributing to inflammatory cell infiltration, matrix deposition, and fibrosis.33 The proinflammatory cascade is mediated by mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways.32,34
Light chains with a strong affinity for Tamm-Horsfall protein form casts that obstruct the flow of tubular fluid and may rupture the tubular epithelium leading to interstitial inflammation. MIDD cases are generally associated with kappa light chains of the 1, 3, or 4 subtype, which may be fragmented or abnormally large and may have atypical glycosylation or amino acid patterns that lead to misfolding, insolubility, and precipitation.35 In contrast, most Ig amyloid cases result from lambda light chains, particularly the lambda-6 subtype.36 Amino acid substitutions within the variable region of the amyloidogenic light chain mediate electrostatic aggregation with serum amyloid P protein, heparan sulfate proteoglycan and other substances, which results in a β-pleated sheet.37 Light chains from patients with AL amyloidosis undergo endocytosis at the glomerulus and subsequent delivery to lysosomes, where amyloid formation occurs, whereas those from patients with glomerular MIDD appear to induce a fibroblast phenotype in mesangial cells, which results in matrix deposition.38 Despite all that is known about the characteristics of pathogenic Ig, the risk for kidney injury in a particular patient with plasma cell malignancy cannot be reliably predicted by analyzing the monoclonal Ig present.
Detection of monoclonal Ig
The identification of monoclonal Ig in serum or urine is critical to the diagnosis of plasma cell malignancies. Protein electrophoresis is inexpensive but has relatively poor sensitivity for free light chains (FLCs) and cannot reliably differentiate monoclonal from polyclonal light chain expansion. Immunofixation is more sensitive than protein electrophoresis but does not quantify the amount of monoclonal Ig present and thus is of limited use in monitoring disease progression and response to treatment.6,39 The newer, nephelometric FLC immunoassay detects both monomers and dimers of kappa and lambda at concentrations less than 2 to 4 mg/L, without confounding from intact Ig.40 The FLC assay does not directly detect clonality, but suggests it through an increase or decrease in the kappa:lambda ratio. Kappa and lambda FLC levels may be elevated in patients with inflammatory diseases such as lupus and in those with CKD, but the kappa:lambda ratio is usually normal.39 In newly diagnosed myeloma, high serum FLC levels are associated with an increased risk of kidney injury.41 Among patients with MIDD, amyloid, or “nonsecretory” myeloma, in whom no monoclonal Ig has been identified with electrophoretic techniques, a substantial proportion will have an abnormal serum kappa:lambda ratio by the serum FLC assay.39,42 The short half-life of light chains and the quantitative nature of the serum FLC assay make it useful for monitoring the activity and response to therapy of light chain myeloma. The FLC assay is not without analytic pitfalls. When FLC quantities are very high, for example, the assay may yield paradoxically normal results because of the antigen excess, a problem common to nephelometric techniques.43 The use of the assay in urine is problematic because variability in the filtration and tubular handling of light chains have confounded attempts to define a normal range.44 Optimal strategies to combine the FLC assay with traditional techniques remain uncertain and at this juncture, the FLC assay complements rather than substitutes for traditional detection techniques.45
Management
When a patient with acute kidney injury and serum or urine monoclonal Ig presents, kidney biopsy data are typically not available and the relative contribution of Ig- and non-Ig–mediated mechanisms is uncertain. Because tubular injury is the most common underlying pathologic pattern, intravascular volume should be restored and hemodynamics optimized to ensure a steady flow of urine in non-oliguric patients if possible, avoiding the use of loop diuretics. Alkalinization of the urine is not of proven benefit and may increase the risk of metastatic calcium-phosphate deposition in the kidney and elsewhere, particularly if hypercalcemia or hyperphosphatemia are present, but repletion of bicarbonate may be necessary regardless if significant bicarbonate wasting is apparent. Hypercalcemia should be treated with intravenous saline and bisphosphonate therapy (dosed for impaired renal function). Allopurinol is effective for hyperuricemia, with rasburicase or hemodialysis reserved for uric acid levels in excess of 15 to 20 mg/dL. Nonsteroidal anti-inflammatory drugs and renin-angiotensin inhibitors must be avoided, and iodinated contrast used only if absolutely necessary, with accompanying prophylaxis. Basic medical management will correct modest degrees of renal impairment in a substantial fraction of incident patients. Additional management options are summarized in Table 2.
Systemic therapy
Documentation or strong suspicion of renal deposition of monoclonal Ig requires prompt antiplasma cell therapy when renal function is impaired. Given the rapid progression and irreversible nature of monoclonal Ig-induced kidney injury, chemotherapeutic agents that act rapidly are preferred. Even dialysis-dependent patients benefit from systemic therapy, and a substantial minority of patients may experience long-term survival with reasonable quality of life.46 Although a comprehensive discussion of myeloma therapy is beyond the scope of this review, survival is improving as a result of novel agents, and those therapies that are particularly relevant to patients with kidney injury are discussed.47 In the future, new biomarkers of kidney injury may be helpful in the development of renoprotective, novel antiplasma cell agents.48
The reversible, first in class proteasome inhibitor bortezomib is a U.S. Food and Drug Administration–approved small molecule boronic acid–derived peptide that targets protein handling mediated by the ubiquitin proteasome pathway. Bortezomib has emerged along with high-dose dexamethasone as among the most effective approaches for treating myeloma when associated kidney injury is present. It is also now well established as therapy for relapsed myeloma and has recently received approval for initial therapy.49,50 Rapidly acting, it is characterized by a median time to best response of approximately 30 days in previously treated patients.49 As malignant plasma cells synthesize and assemble large quantities of Ig, they are particularly susceptible to proteasome inhibitors, which induce myeloma cell apoptosis in part by interfering with protein handling.51,52 Bortezomib also inhibits the NF-κB and MAPK pathways, which leads directly to myeloma cell apoptosis while also disrupting myeloma-stromal cell interactions and tumoral angiogenesis.53 Importantly, by inhibiting the NF-κB and MAPK pathways, bortezomib mitigates the inflammatory state induced in proximal tubule cells by excessive light chain handling and so may protect these cells from apoptosis by up-regulating heat shock and other survival-associated proteins.54,55 In contrast to traditional agents whose toxicity increases in renal impairment, the antimyeloma effects and safety profile of bortezomib appear unchanged in this setting, and renal dose adjustment is therefore not required.56,–58 When combined in various regimens with dexamethasone, melphalan, doxorubicin, or thalidomide, 40% to 50% of patients who respond to therapy will experience significant recovery of renal function within 2 to 3 weeks.58,,,,–63 Improvement in renal function appears to precede the expected antiplasma cell effects of bortezomib, possibly reflecting anti-inflammatory properties of the agent.64 Bortezomib may also be useful for a range of other nephrologic conditions mediated by long-lived plasma cells, including antibody-mediated kidney allograft rejection, with studies ongoing in this regard.65
High-dose dexamethasone regimens also act rapidly and can have similarly dramatic success in reversing renal impairment.66 To improve efficacy of dexamethasone, it is often combined with thalidomide or its potent, newer derivative, lenalidomide, which interferes with myeloma cell growth, disrupts myeloma and bone marrow stromal cell interactions, down-regulates promyeloma cytokines, and up-regulates antimyeloma T-cell activity.67 Although the pharmacokinetics of thalidomide appear unaffected by renal function, hyperkalemia has been reported in the setting of renal dysfunction.68,69 Dose reduction is required with lenalidomide, as the drug's clearance falls significantly in advanced CKD.70 Importantly, patients with creatinine in excess of 2.5 mg/dL have been excluded from lenalidomide trials, and patients with lesser degrees of renal dysfunction experience more adverse events, particularly myelosuppression.71 Although further study is clearly necessary, clinical experience suggests that dose-reduced lenalidomide can be safely and effectively administered in the setting of renal impairment. Thalidomide, lenalidomide, and high-dose dexamethasone are associated with thromboembolic disease, which in turn may complicate their administration to patients with nephrotic syndrome and those receiving erythropoietin, both of which may also predispose to thrombosis.
Given that these novel agents have different mechanisms of action, their use in combination appears to result in synergistic antimyeloma effects, with some expected overlapping toxicity. Dexamethasone in combination with thalidomide is noncytotoxic and has proven particularly useful for high risk patients with renal failure who require significant cytoreduction before SCT.72 Bortezomib combined with thalidomide and dexamethasone (so-called VTD) and other agents in a tandem-transplant scheme has also proven superior to earlier combinations.73
Stem cell transplantation
SCT remains a mainstay of myeloma therapy and the availability of novel therapeutic approaches has enhanced its effectiveness while also prompting active research about its optimal timing and role.74 Patients with myeloma and renal failure can successfully receive SCT with an appropriately dose-reduced conditioning regimen, but toxicity is increased.75 Randomized trials have excluded patients whose serum creatinine exceeds approximately 2.3 mg/dL, and thus the benefits of SCT in patients with more significant renal failure are unproven.76 Autologous SCT has been used successfully in patients with Ig amyloid and MIDD, though treatment related mortality is high when systemic disease is present and in the case of amyloid, patient selection has been suggested as an explanation for the apparent advantage of SCT over conventional therapy.77 In addition, melphalan conditioning has been associated with acute kidney injury in patients with amyloid undergoing SCT.78 Although 90% of myeloma patients who undergo autologous SCT will eventually relapse, allogeneic SCT offers a potential cure because of the graft-versus-myeloma effect, with acceptable treatment related mortality when reduced intensity conditioning is used.79 Small numbers of patients with myeloma and end-stage kidney disease have successfully been treated with nonmyeloablative conditioning followed by simultaneous kidney and allogeneic SCT from an HLA-identical sibling, in some cases achieving complete remission of myeloma as well as stable kidney allograft function without a requirement for immunosuppression because immune cells from the allograft bone marrow do not reject a kidney from the same donor.80
Plasmapheresis
The value of plasma exchange in monoclonal Ig-associated kidney injury remains uncertain. The high volume of distribution of light chains and IgG results in low clearance relative to body stores, and rapid plasma refill occurs after each pheresis session.81 Initial clinical studies provided conflicting results and the largest randomized controlled trial of more than 90 patients showed no benefit of plasmapheresis compared with usual care.82 Kidney biopsy procedures were not required for entry into this latter study, however, and the high recovery rate (40%) in the control group suggests that many of the patients had kidney injury at least in part from Ig-independent mechanisms, thus obscuring the potential benefit of pheresis. An uncontrolled, retrospective analysis claimed a benefit to pheresis in the subset of patients with cast nephropathy whose light chain burden was reduced by more than 50%.83 Pheresis remains the standard of care in cases of hyperviscosity syndrome (seen with IgA or IgG3 substypes) or IgM immunoglobulin. A European-based randomized controlled trial of plasmapheresis in myeloma (MERIT, Myeloma Renal Impairment Trial; http://www.ncrn.org.uk/) ended enrollment in early 2009, and dialytic techniques to remove nephrotoxic light chain are also under study.84
Kidney transplantation
Myeloma appears to be more prevalent in dialysis patients than in the general population, possibly due to enrichment of the dialysis population with patients who have sustained myeloma-associated kidney failure.85 Kidney transplantation is occasionally contemplated as an alternative therapy to dialysis and yet multiple factors including the risk of myeloma recurrence and infection conspire against the success of transplantations in patients with plasma cell malignancies. The risk of monoclonal Ig-mediated allograft dysfunction among patients undergoing transplantation is low among patients who remain in remission if the original kidney lesion was cast nephropathy, but high among patients who had MIDD.86,87 Early, severe allograft dysfunction as a result of a monoclonal Ig-mediated necrotizing and crescentic glomerulonephritis has been reported.88 Despite the high prevalence of MGUS, there are only a handful of reported cases of kidney transplantation in such patients and concern remains that the progression to myeloma may accelerate in the context of maintenance immunosuppression.89
To be considered for kidney transplantation, most centers require that patients with myeloma achieve and maintain a durable remission, typically for 3 to 5 years. For all forms of plasma cell malignancy, including MGUS, monoclonal Ig levels must be low and stable before transplantation, Pretransplantation informed consent must address the risk of early graft loss from recurrent disease and the risk that transplant immunosuppression will accelerate the underlying malignancy or premalignant condition.86 Kidney transplantation is feasible in highly selected patients with MIDD and amyloid who achieve complete hematologic remission from SCT.90 Interestingly, both bortezomib and lenalidomide have been used successfully to treat kidney allograft MIDD, and with the impact of novel therapies in myeloma continuing to expand, the potential of newer combinations in this setting conferring benefit to patients is considerable.91
Conclusions
Plasma cell malignancies are commonly associated with kidney injury, often as a result of nephrotoxic monoclonal Ig. Traditional electrophoretic techniques remain the gold standard for identifying monoclonal Ig, whereas the serum FLC assay provides an additional tool for identifying and monitoring monoclonal Ig levels, particularly FLCs. Bortezomib, lenalidomide, and other agents appear to be more effective and selective for plasma cells and their microenvironment, with clear superiority now evident over traditional chemotherapy, and bortezomib appears to have a particular role in patients with myeloma-associated kidney injury. Autologous SCT remains a critical part of myeloma management but its use is limited in patients with kidney disease and its overall role will continue to evolve as systemic therapy improves. The value of plasma exchange for myeloma-associated kidney injury is an area of active research. Kidney transplantation is an option only in well-selected patients who have enjoyed prolonged remission and have low monoclonal Ig levels. Wider use of kidney biopsy to identify monoclonal protein deposition in patients with mild degrees of kidney dysfunction may be indicated as therapeutic options improve.
Acknowledgments
This study was supported in part by a grant from AstraZeneca (N.S.R.), a grant from Genzyme (B.D.H.), and grants from Celgene, Novartis, and Millennium (K.C.A.).
National Institutes of Health
Authorship
Contribution: E.C.H. performed the initial literature search and wrote the initial manuscript; P.G.R. provided intense review and expertise and multiple other references; and N.B.G., T.R.S., N.S.R., B.D.H., and K.C.A. contributed knowledge, expertise, references, and editorial assistance with the manuscript.
Conflict-of-interest disclosure: N.S.R. reports receiving consulting/advisory board fees from Amgen, Celgene, and Novartis. K.C.A. reports receiving consulting/advisory board fees. P.G.R. reports receiving consulting/advisory board fees from Millennium, Celgene, and Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Eliot C. Heher, Nephrology Division, Massachusetts General Hospital, 165 Cambridge St, Boston, MA 02114; e-mail: eheher@partners.org.