Abstract
In this double-blind, placebo-controlled study, 363 patients with untreated multiple myeloma were randomized to receive either melphalan-prednisone and thalidomide (MPT) or melphalan-prednisone and placebo (MP). The dose of melphalan was 0.25 mg/kg and prednisone was 100 mg given daily for 4 days every 6 weeks until plateau phase. The dose of thalidomide/placebo was escalated to 400 mg daily until plateau phase and thereafter reduced to 200 mg daily until progression. A total of 357 patients were analyzed. Partial response was 34% and 33%, and very good partial response or better was 23% and 7% in the MPT and MP arms, respectively (P < .001). There was no significant difference in progression-free or overall survival, with median survival being 29 months in the MPT arm and 32 months in the MP arm. Most quality of life outcomes improved equally in both arms, apart from constipation, which was markedly increased in the MPT arm. Constipation, neuropathy, nonneuropathy neurologic toxicity, and skin reactions were significantly more frequent in the MPT arm. The number of thromboembolic events was equal in the 2 treatment arms. In conclusion, MPT had a significant antimyeloma effect, but this did not translate into improved survival. This trial was registered at www.clinicaltrials.gov as #NCT00218855.
Introduction
In recent years, there has been considerable progress in the treatment of multiple myeloma. Thalidomide was demonstrated to be effective in patients with relapsing multiple myeloma in 1999.1 This discovery generated an array of studies that confirmed the initial results that approximately 30% of patients with relapsing multiple myeloma respond to thalidomide.2 A further step was to test the effect of thalidomide in newly diagnosed multiple myeloma; and in 2000 to 2001, several groups in Europe launched randomized studies with similar design, aimed at patients who had not received previous treatment and were not eligible for high-dose therapy. An Italian randomized study with melphalan, prednisone, and thalidomide (MPT) versus melphalan and prednisone (MP) demonstrated a significant difference in progression-free survival (PFS),3 but this did not translate into an overall survival (OS) benefit.4 A French study demonstrated a significant difference in both PFS and OS in patients 65 to 75 years of age,5 and the same result was found in a separate study in patients older than 75 years.6 A Dutch study has demonstrated a difference in event-free survival but no difference in OS.7
In the Nordic countries, we started a phase III trial in 2002 in which 363 patients not eligible for high-dose treatment with stem cell support were randomized to receive either MPT or MP and placebo. In this report, we present the results of this study.
Methods
Patients
Criteria of inclusion in the study included previously untreated symptomatic multiple myeloma in patients who were not eligible for high-dose treatment with autologous stem cell support. A diagnosis of multiple myeloma was based on these criteria: criterion A (monoclonal Ig > 30 g/L or IgA > 20 g/L, IgD, or IgE of any concentration in serum and/or κ- or λ-light chain > 1 g/24 hours in urine); criterion B (M protein at a lower concentration than in criterion A); criterion C (10% or more of plasma cells in the bone marrow or a plasma cell tumor in bone or soft tissue); and criterion D (osteolytic destructions in the skeleton). To make a diagnosis of multiple myeloma, the combination of A + C, A + D, B + C + D or, in case of nonsecretory multiple myeloma, C + D was required. Durie-Salmon stage I to III and performance status World Health Organization (WHO) 0 to 4 were accepted.
Women of childbearing age, patients with psychiatric disease, and those expected to survive less than 3 months were excluded, as were patients who did not cooperate or who refused consent. Patients were recruited from 48 hospitals in Norway, Sweden, and Denmark from January 2002 to May 2007.
Study design
Patients were randomized to receive either MPT or MP with placebo. Randomization was centralized and performed by telephone call or fax. The randomization was stratified according to WHO performance status (≤ 2 or 3-4) and β2-microglobulin (≤ 2.6 mg/L, > 2.6 mg/L, or not known). The primary endpoint of the study was OS. Secondary endpoints were PFS, response rate, time to progression health-related quality of life, and toxicity. All patients gave written informed consent in accordance with the Declaration of Helsinki. The study was approved by ethics committees and authorities in Norway, Sweden, and Denmark. All authors had access to primary clinical trial data.
A safety committee with 3 independent members evaluated the study when 150 patients had been observed for 6 months. Results and toxicity were found acceptable by the committee. The study was unblinded in November 2007. The evaluation of outcomes was performed without knowledge of treatment arm.
The dose of melphalan was 0.25 mg/kg, and the dose of prednisone was 100 mg daily for 4 days every 6 weeks. Cycles of treatment were continued until plateau phase. The dose of thalidomide/placebo was 200 mg daily for one week and thereafter 400 mg daily. This dose was maintained until plateau phase and thereafter continued at 200 mg daily. Dose reduction was allowed if necessary because of side effects. The study drug was stopped, and further treatment was decided by the investigator in the event of primary resistance, intolerable side effects, or withdrawal of consent. The recommendation in the protocol was to repeat the induction treatment at first relapse and continue maintenance treatment until second relapse. However, most investigators stopped the study drug at first relapse. No routine prophylaxis for venous thrombosis was recommended. Thalidomide and placebo were provided by Grünenthal and stored in a central pharmacy in each of the countries. The content of thalidomide in the tablets was assayed twice during the study and was found to be unchanged (97.5-99.5 mg thalidomide per tablet; DB Lab). Melphalan and prednisone were ordered from the pharmacies as standard treatment. Whether thalidomide or placebo was given was unknown to the patient, investigator, and monitor. Response and relapse were evaluated by the investigator and reevaluated by the working group for the study in several meetings.
Laboratory investigations included hemoglobin, white blood cell count, platelets, creatinine, calcium, albumin, alanine aspartate transaminase, and prothrombin time-international normalized ratio. Patients were monitored with blood samples, clinical evaluation, and response evaluation every 6 weeks during the first 6 months, and thereafter every third month. Bone marrow aspirate was taken at diagnosis and thereafter on demand or to confirm a complete remission.
Health-related quality of life was measured by the validated European Organization of Research and Treatment of Cancer QLQ-C30 questionnaire, which was completed by the patient at inclusion and later posted to the patient every third month throughout the study.
In 2006, it was decided to send out a questionnaire for 328 patients to all investigators asking whether thromboembolic events had been observed. It was also asked whether and for which time periods the patients were using warfarin, heparin, salicylates, or erythropoietin.
Definitions
Partial response (PR) was defined as more than 49% reduction of M protein in serum and more than 89% reduction of M protein in urine or to a level of less than 0.2 g/24 hours. In addition, a more than 49% reduction of size of soft tissue plasmacytoma and no increase of size or number of bone lesions was required for PR. If a reduction of M protein was difficult to observe because of low initial concentration, a reduction in the number of plasma cells in a bone marrow smear could be used. Very good partial remission (VGPR) was defined as more than 89% reduction of M protein in serum and in urine to less than 100 mg/24 hours. Complete response (CR) was defined as no detectable M protein in serum or urine as assayed by electrophoresis, less than 5% plasma cells in bone marrow, total regression of soft tissue plasmacytoma, and no increase in size and number of bone lesions. Immunofixation was not required. Minor response (MR) was defined as 25% to 49% reduction of M protein in serum and a 50% to 89% reduction of M protein in urine, but not to a level of less than 0.2 g/24 hours. In addition, a 25% to 49% reduction of size of soft tissue plasmacytoma and no increase in size or number of bone lesions were required for MR. For all categories of response, we considered that new vertebral compression fractures did not exclude response. Responders had to show clinical improvement, no need of transfusions, normal serum calcium, and no increase of creatinine. Nonresponse (NR) was defined as not meeting the criteria for CR, PR, MR, or PD. The best response during the first 12 months was recorded.
Plateau phase was defined in patients achieving CR, PR, or MR as either no detectable M protein or less than 10% variation in 3 consecutive analyses of M protein in serum, or 3 analyses of M protein in urine with values less than or equal to 0.2 g/24 hours. After response, progressive disease (PD) was defined as at least 2 consecutive analyses (first taken as date of relapse) with an increase in M protein in serum of more than 24% (to at least 10 g/L) or an increase in M protein in urine of more than 24% (to at least 1 g/24 hours), or any other unequivocal sign of disease progression (hypercalcemia, increase of osteolytic foci or plasmacytoma, or increase in number of plasma cells in the bone marrow). A similar definition was used for progression from NR. In addition, patients with NR or MR were recorded as having PD when new treatment was started, irrespective of concentration of M protein.
PFS was calculated from the date of randomization until the date of progress/relapse or death of any cause. Time to progression was calculated from the date of randomization until progression/relapse.
Statistical analysis
The sample size needed to detect a hazard ratio of 1.4 was calculated; this corresponds to anticipated median survival of 28 months (MP) versus 40 months (MPT). For 80% power and a type I error of 5%, 282 events (deaths) are required. Thus, it was planned to recruit 800 patients (400 in each group), with analyses taking place at 40 months after trial initiation.
In the event, fewer patients were recruited (175 MP, 182 MPT). By December 2008, 209 deaths had occurred, whereas we estimated 282 in the protocol. This means that the effective power is reduced to 72% or, equivalently, that we have 80% power of detecting a hazard ratio of 1.48 instead of 1.4 as specified in the design. For PFS, 272 events were observed, providing 79% power to detect a ratio of 1.4
Survival curves were consistent with proportional hazards, and so Cox models were used on an intention-to-treat basis with covariate adjustment considered for stage of disease, performance status, age, and sex. The results of these analyses were confirmed using log-rank tests.
The patients' self-assessments of quality of life using the QLQ-C30 were analyzed using standardized area under the curve. Generalized estimating equations were also used; these make full use of the repeated quarterly (every 3 months) measures and allow for the within-patient correlations over successive time points. Analyses were carried out first using the observed values of the QLQ-C30 scores, and then with baseline (prerandomization) QLQ-C30 scores as covariates; this second analysis is equivalent to examining change from baseline for each patient. Physical functioning, from the QLQ-C30, was prespecified in the protocol as being the primary health-related quality of life outcome of interest. The randomization stratification factors (WHO and β2-microglobulin) were also used as covariates, together with baseline characteristics (International Staging System [ISS], age, and sex) when they were found to be significant prognostic indicators of outcome. Because the results were in all cases similar, only the analyses adjusted for baseline QLQ-C30 score are presented. To further investigate the impact of potential bias from missing data, multiple imputation was explored using the ICE and MIM programs and predictive factors.8,9
All analyses were carried out using STATA version 10.0 (Stata Statistical Software, Release 10, StataCorp LP).
Results
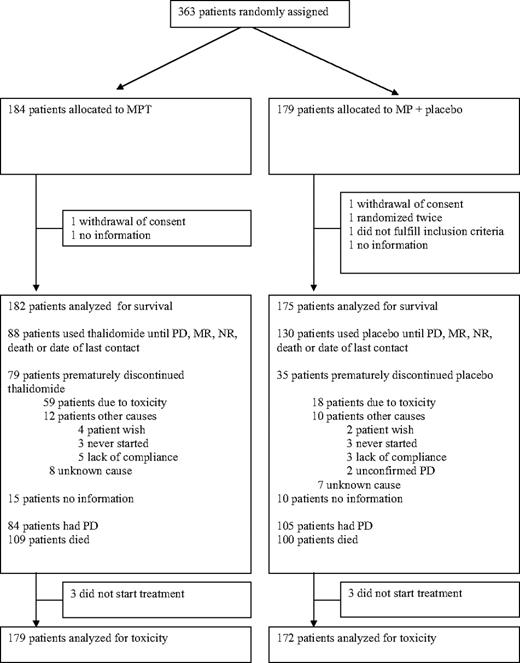
A total of 363 patients were included and randomized. Six patients were excluded from analysis as shown in Figure 1; 2 withdrew consent before treatment, and 6 were lost to follow-up. In addition, one duplicate randomization was excluded. A total of 357 patients were analyzed: 182 patients in the MPT arm and 175 patients in the MP arm (Figure 1). Among these, 25 patients were not evaluable for response in the MPT arm and 13 patients in the MP arm because of early exclusion (4 MPT, 3 MP), early death (12 MPT, 8 MP), and missing follow-up data (9 MPT, 2 MP). These patients were included in the survival. Information about length of treatment and discontinuation is given in Figure 1. Baseline characteristics of the patients are shown in Table 1.
All patients were followed up with respect to response and toxicity until November 2007 (6 months after the last inclusion), and 18 additional months for survival (ie, until December 2008). The median follow-up time, defined as time from randomization to last follow-up with censoring for death, was 42 months.
Survival
OS is shown in Figure 2, and there is no statistically significant difference (P = .16, Cox model with covariates, P = .35, log-rank test ignoring covariates). Median survival was 29 months (95% confidence interval [CI], 25-38 months) in the MPT arm and 32 months (95% CI, 27-38 months) in the MP arm. There was no difference in PFS between the 2 arms (Figure 3), with medians of 15 months (95% CI, 12-19 months) for MPT and 14 months (95% CI, 11-18 months) for MP. In those with reported progression, the median time to progression was 13 months (95% CI, 11-15 months) in the MPT arm and 12 months (95% CI, 10-14 months) in the MP arm (P = .84, log-rank test) (data not shown).
Inspection of the survival curves suggested a slight increase in mortality in the thalidomide arm during the first 6 months. In this period, there were 35 deaths in the MPT arm versus 21 deaths in the MP arm; however, even ignoring the fact that this was a post hoc analysis, this difference is not statistically significant. By further analyses, it was observed that the increase in deaths was mainly among patients older than 75 years (Figure 4). In this group, 23 died in the MPT arm and 12 died in the MP arm. Below 75 years of age, the corresponding figures were 12 and 9 deaths. We classified the cause of death without knowing the treatment allocation. Above 75 years of age, 8 patients died from infection, 6 died of unknown cause (no information, uncertain classification), and 6 died of progressive disease in the MPT arm, whereas the corresponding numbers in the MP arm were 3, 1, and 4 patients.
There was no evidence of age (older/younger than 75 years) interaction on PFS (P = .31), on OS (P = .92), or on time to progression (P = .75). Similar results were observed for ISS and β2-microglobulin.
Response rates
Best responses obtained in the first 12 months are shown in Table 2. In the MPT group, 57% had at least PR and 23% had at least VGPR, whereas the corresponding number was 40% and 7% in the MP group (χ2 for trend, P < .001).
Quality of life
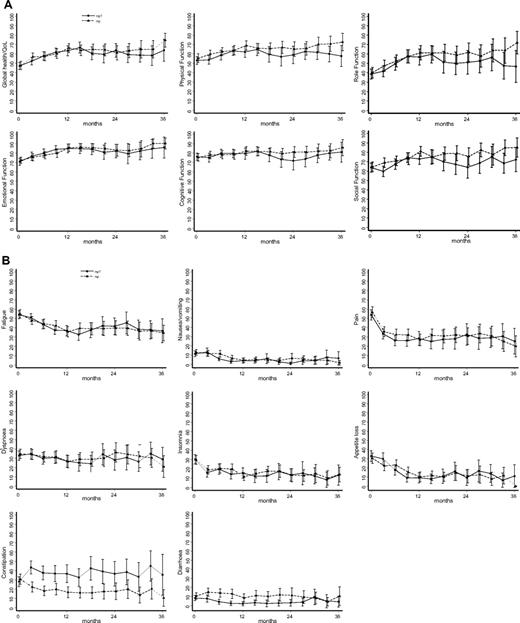
Compliance with completing the QLQ-C30 was 82% in the MPT arm and 90% in the MP arm at 3 months and 50% and 62%, respectively, at 12 months. Figure 5 shows the mean QLQ-C30 scores only for those patients who returned completed questionnaires. In both treatment arms, the quality of life improved after initiation of treatment. There was a marked increase in constipation among patients in the MPT arm (P < .001, analyses by generalized estimating equations), with a corresponding slight tendency to diarrhea in the MP patients (P = .002). There were borderline significant differences in physical function (P = .025) and social function (P = .013). Comparable results were obtained from analyses using standardized area under the curve, confirming the robustness of the analyses. In an attempt to check whether there was probably bias arising because of the poor compliance, the analyses were repeated using multiple imputation; the revised P values were .001 (constipation), .033 (diarrhea), .14 (physical function), and .024 (social function).
Self-reported quality of life during 36 months. (A) Global parameters. (B) Specific health parameters. Data are mean values and 95% confidence intervals. QL indicates global quality of life; RF, role function; PF, physical function; SF, social function; EF, emotional function; CF, cognitive function; DI, diarrhea; CO, constipation; AP, appetite loss; NV, nausea/vomiting; DY, dyspnea; SL, sleep loss; PA, pain; FA, fatigue; and FI, financial problems.
Self-reported quality of life during 36 months. (A) Global parameters. (B) Specific health parameters. Data are mean values and 95% confidence intervals. QL indicates global quality of life; RF, role function; PF, physical function; SF, social function; EF, emotional function; CF, cognitive function; DI, diarrhea; CO, constipation; AP, appetite loss; NV, nausea/vomiting; DY, dyspnea; SL, sleep loss; PA, pain; FA, fatigue; and FI, financial problems.
Dose of thalidomide
The actual dose of thalidomide and placebo taken at day 30, day 90, and at one year is given in Table 3. The intended dose was 400 mg for patients before plateau phase and 200 mg after plateau phase had been reached. Both these categories of patients are included in the table. The calculations demonstrate that an increased number of patients had to discontinue thalidomide, as well as a marked reduction in actual dose of thalidomide taken. We analyzed the doses taken according to age and did not find any indication that patients older than 75 years took lower doses or stopped taking thalidomide compared with patients younger than 75 years (data not shown). Median duration of thalidomide treatment for patients living longer than one year was 236 days.
Relapse treatment
The choice of therapy after relapse is known for 71 of 82 (87%) patients in the MPT arm and 87 of 104 (84%) in the MP arm (Table 4). A total of 45% of the patients in the MP arm received thalidomide-based chemotherapy at first relapse or later, whereas the corresponding number was 23% in the MPT arm. Bortezomib was given to 18% and 20% and lenalidomide to 3% and 2% in the MP and MPT arms, respectively.
Toxicity
The adverse events that were significantly more frequent in the MPT arm are shown in Table 5. In addition, somnolence/fatigue was more frequent in the MPT arm, however, with borderline significance (30% grade 1 or 2, 8% grade 3 or 4 vs 26% and 4% in the placebo group, P = .080). The incidence of grade 3 or 4 toxicity for hemoglobin levels was 4% versus 8%, for granulocytes 25% versus 20%, for platelets 8% versus 11%, for infections 15% versus 10%, and for cardiologic toxicity 7% versus 5% in the MPT arm versus the MP arm. The differences were not statistically significant. There were equal numbers of cardiac infarction and heart insufficiency in both arms.
A total of 59 patients discontinued thalidomide resulting from toxicity, whereas 18 patients discontinued in the placebo group (Figure 1). The side effects that most frequently led to discontinuation of thalidomide were polyneuropathy in 13 patients, constipation in 8 patients, skin reactions in 7 patients, somnolence in 7 patients, and dizziness in 6 patients (included in nonneuropathy neurologic toxicity). The corresponding number of patients in the MP arm were 0, 1, 0, 0, and 2.
Thromboembolic events
The incidence of thromboembolic events was analyzed both by ordinary case report forms and by a separate questionnaire. The investigators responded to the questionnaire from 243 (75%) patients: 123 in the MPT arm and 120 in the MP arm. The number of patients with thromboembolic events was altogether 15 (8%) in the MPT arm and 14 (8%) in the MP arm. Of these, 12 patients were spontaneously reported in the MPT arm and 7 patients in the MP arm. It was altogether registered 14 patients with deep vein thrombosis (DVT), 14 patients with pulmonary embolism, and one patient with both. Median time to the thromboembolic event was 64 days (range, 3-1509 days) in the MPT arm and 280 days (range, 0-743 days) in the MP arm (not significant). No antithrombotic prophylaxis was recommended in the study.
By the questionnaire, we also obtained information of the number of patients using anticoagulants for other reasons or erythropoietin. Salicylates were used by 24% and 23% and warfarin was used by 15% and 17% in the MPT and MP arms, respectively. Altogether, 39% and 40% of the patients in the 2 treatment arms used drugs that may have prophylactic effect on thromboembolic events. Erythropoietin was used by 11% in the MPT arm and 16% in the MP arm (not significant).
Discussion
In this randomized, placebo-controlled study, we found that addition of thalidomide to standard MP therapy in elderly myeloma patients had a significant antimyeloma effect in terms of increased proportions of high-quality responses. Despite this, we found no significant impact on PFS or OS.
The optimal dose of thalidomide is not established. In the studies that introduced thalidomide in relapsing myeloma, the intended dose was 800 mg, which later was shown to be too high a dose.1 In the present study, we aimed at a dose of 400 mg in the treatment phase and 200 mg in the maintenance phase. However, during the course of the study, there was an increasing understanding that lower dose was sufficient for optimal effect. In addition, the potency of thalidomide became more evident in terms of side effects; and as a consequence, the actual dose taken tended to be reduced. By 3 months, fewer than 20% of patients were taking 400 mg of thalidomide, and 28% had stopped treatment. This dose was calculated from patients with active treatment (planned dose, 400 mg) as well as from patients in maintenance phase (planned dose, 200 mg). In the MP arm, the corresponding percentages were 50% and 12%, confirming the need for considerable dose reduction in the thalidomide arm.
MPT has been compared with MP in 5 randomized trials: an Italian study,3 2 French studies in patients younger than 75 years5 and patients 75 years of age and older,6 a Dutch study,7 and the present Nordic study. All studies have reported significant improvement of response rate by the addition of thalidomide. However, there are differences regarding impact on survival. In the 2 French and the Dutch studies, OS and PFS were significantly increased. In the Italian study, PFS or event-free survival, but not OS, was significantly improved. In the Nordic study, neither PFS nor OS was improved.
Certain aspects that may have significance for the differences between the studies are discussed here. There were differences in inclusion criteria between the studies, which was reflected in particular by the fact that 30% of the patients in our study had WHO performance status 3 or 4 compared with 6% to 8% and 6% in the Italian3 and French5,6 studies. The proportion of patients with WHO performance status more than 3 in our study is similar to our earlier population-based, unselected, and nearly complete registration studies.10
In addition, the scheduled dose of melphalan and thalidomide varied considerably between the studies. However, none of the studies was designed for evaluating optimal dose of thalidomide, and it is difficult to draw detailed conclusions about dose or duration of treatment and effect across the studies. We notice that beneficial effect has been observed at a dose of 100 mg daily, indicating that a dose in the lower range is sufficient
The median age in all studies was quite similar, ranging from 70 to 74 years in 4 of the studies.3,5,7 Age alone is therefore probably not of major importance. This is further underlined by the French study on patients older than 75 years, which demonstrates a survival benefit of thalidomide.6
In conclusion, patient selection and, in particular, differences in proportion of WHO performance status more than 3, differences in dose and schedule of thalidomide and melphalan may have impact on the result. Comorbidity that is common in the older age groups may influence the WHO performance status. One might speculate whether high doses of thalidomide and inclusion of patients with poor performance status may at least to some extent explain the lack of translation from good responses to improved PFS or OS in our study.
We noted in a retrospective analysis that there was an increased number of deaths in the early phase of thalidomide treatment in patients older than 75 years. It is of interest that a similar increase of early deaths was observed in the Italian study3 and Dutch study7 as well as in a study comparing thalidomide-dexamethasone with MP.11 The early phase of treatment appears to be vulnerable, and selection of patients to receive thalidomide may be of importance. A practical conclusion is to use thalidomide at doses of 100 to 200 mg and exclude patients or reduce dose in patients with age older than 75 years and WHO performance status 3 or 4.
Thalidomide plus dexamethasone has also been evaluated in phase III clinical trials.11–13 Thalidomide plus dexamethasone produced better responses compared with dexamethasone12,13 or MP,11 whereas survival was reduced compared with MP.11 In the studies comparing thalidomide plus dexamethasone with dexamethasone alone, there was improvement in time to progression and PFS,12 but not in OS.12,13 However, these studies were not powered to detect differences in survival.
The proportions of patients who discontinued thalidomide increased gradually from 22% at one month to 56% at one year. However, the specific thalidomide-related discontinuation was lower as 9% and 33% of the patients discontinued the placebo drug at the corresponding time points. Patients who had died or relapsed were excluded from this analysis (ie, only patients expected to be taking the study drug or placebo were evaluated). The discontinuation of the drug was therefore assumed to be related to thalidomide side effects or suspicion of this. Evaluation of the dose taken demonstrates that the 400-mg dose of thalidomide was most often reduced compared with placebo. Discontinuation of treatment resulting from toxicity has been reported to be 31.6% after a median of 2.2 months in the Italian study and 45% and 42% in the 2 French studies. In the Dutch study, only 28% received the full dose of thalidomide after 6 cycles. All studies demonstrate that a considerable proportion of patients have to stop treatment with thalidomide. However, many patients taking placebo also discontinue treatment. Our study specifically demonstrates a poor compliance for the 400-mg dose of thalidomide.
We found significant differences in adverse events for neuropathy, constipation, nonneuropathy neurologic toxicity, and exanthema and borderline significant difference for somnolence/fatigue. Similar toxicity profile has also been reported by others. We notice that our report differs somewhat from the French study, which also was blinded.6 In this study, they observed significant difference for neuropathy and grade 3 or 4 neutropenia, whereas we could not confirm the latter.
During the study, it was reported by other investigators that there was an increased incidence of DVT in patients taking MPT. We therefore considered introducing prophylactic treatment for thromboembolism. However, the total number of DVTs was low in our study, and we explored this further by sending a questionnaire to all investigators in 2006. Surprisingly, the reported incidence of thromboembolic events was 8% and equal in both treatment arms. Most studies on combination treatment with thalidomide report an increased incidence of thromboembolic events.14 In the Italian3 and French5 studies, thromboembolic events were reported to be 20% and 12%, respectively, in patients not receiving prophylaxis in the MPT arm. On the other hand, the double-blind French study6 in older patients reports an overall incidence of 7%; and similar to our study, there was no difference between the treatment arms. Although no prophylaxis was recommended, we found that 40% of the patients in our study were indeed taking drugs with antithrombotic effect, and this may partially explain the low incidence of thromboembolic events.
Quality of life is an important issue, particularly in the treatment of an incurable disease. The use of the validated European Organization of Research and Treatment of Cancer QLQ-C30 questionnaire sent directly to the patients every 3 months, together with the placebo design of the study, allowed us to study quality of life without any reporting bias. The QLQ-C30 measures both global and more specific health parameters. An initial improved quality of life was reported, reflecting response to treatment. Although compliance in returning the questionnaires was poor after the initial couple of assessments, later results were entirely consistent with the (good compliance) patient reports at 3 and 6 months. Multiple imputation also showed no evidence that there was bias in the comparison of treatments. We found a marked difference in constipation between the 2 treatment arms and a smaller but corresponding reverse effect on diarrhea, whereas there were only minor differences in any other outcomes. A limitation of the questionnaire is that it does not include specific questions related to polyneuropathy, although this may partially have been captured in the reported global quality of life. Investigator-reported side effects and patient-reported quality of life are different and complementary approaches to the problem of toxicity and tolerability. Together, they provide a more complete insight into the impact of thalidomide. It was interesting to note the discrepancy between investigator- and patient-reported fatigue. Because fatigue is an expected side effect of thalidomide, the physicians may have overestimated it in patients who they suspected of being in the thalidomide arm. Similarly, in unblinded trials, the side effects of thalidomide may have been exaggerated.
A major strength of our study is the placebo-controlled design. The study drug was blinded for the patients, the investigators, and the monitors throughout the study until all decisions about response rates and progression had been taken. This design protects against the inevitable bias seen in open-labeled studies. It has been shown that placebo-controlled studies generally find fewer and smaller differences than open-labeled studies.15 However, the French study on patients older than 74 years also had a blinded design and demonstrated a benefit for thalidomide with respect to PFS and OS.6
In conclusion, we report a significantly improved response rate in previously untreated myeloma patients 65 years of age or older by addition of thalidomide to standard MP. However, the study did not confirm previous reports on improved PFS and OS. Patients older than 75 years may be vulnerable in the initial phase of the treatment, and further investigation of this group is indicated. Meanwhile, care should be taken to avoid high doses of thalidomide to frail elderly patients.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lillian Keller, National Hospital, Copenhagen and Karin Tulluan, Norwegian Society of Cancer Research, Trondheim for data management and study nurse Turid Almvik for excellent assistance during the study.
A complete list of Nordic Myeloma Study Group participants appears in the online Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
This work was supported by the Norwegian Cancer Society and the Norwegian Research Council (grant 160388/V50). Thalidomide and placebo were provided by Grünenthal Gmbh, Aachen.
Authorship
Contribution: A.W., P.G., I.T., and G.J. designed the protocol, conducted the study, collected patients, and reviewed all data and the manuscript; A.W. wrote the paper; N.A., L.A., B.B., K.C., I.M.D., K.F., N.G., E.H., Ø.H., M.H., T.K., L.M.K., J.L.N., O.L., U.-H.M., I.N., J.R., M.S., J.H.S., and F.W. discussed the protocol, collected patients, and reviewed the manuscript; F.W. designed and organized the QoL study; and P.F. advised on the statistical design, carried out the statistical analyses, and reviewed the manuscript.
Conflict-of-interest disclosure: A.W. is on the advisory boards of Janssen Cilag, Celgene, and Genzyme. P.G. is on the advisory boards of Janssen Cilag, GenMab, Schering-Plough, and Bristol-Myers Squibb. G.J. is on the advisory board of Merck Serono. B.B. has been employed by Roche since January 1, 2008. K.C. is on the advisory board of Celgene. K.F. has received a fee from Janssen Cilag. The remaining authors declare no competing financial interests.
Correspondence: Anders Waage, Department of Hematology, St Olavs Hospital/NTNU, N-7006 Trondheim, Norway; e-mail: anders.waage@ntnu.no.
References
Author notes
G.J. and I.T. contributed equally to this study.