The matrix metalloproteinase (MMP) MT1-MMP plays pivotal roles in leukocyte physiology such as monocyte diapedesis, dendritic cell migration, and T-cell homing. MT1-MMP is a surface-anchored “master switch” proteinase that cleaves a variety of substrates including extracellular matrix components, matrix receptors, and also other MMPs. However, little is known about the mechanisms enabling intracellular trafficking and exposure of MT1-MMP on the cell surface. We now show that, in primary human macrophages, MT1-MMP–positive vesicles travel bidirectionally along microtubules, in a process regulated by KIF5B and KIF3A/KIF3B kinesins. SiRNA-induced knockdown revealed that transport by KIF5B and KIF3A/KIF3B is crucial for delivery of MT1-MMP to the cell surface and also for surface-associated functions of MT1-MMP, such as shedding of the matrix receptors CD44 and syndecan-1 or degradation of extracellular matrix at podosomes. These data show that kinesin-mediated intracellular transport of MT1-MMP is a pivotal process that allows macrophages to dynamically modify their pericellular environment. These data also identify specific kinesins as potential targets for the early manipulation of MT1-MMP activity in tissues.
Introduction
Modification of the pericellular environment is crucial for leukocyte function under both physiologic and pathologic conditions. This dynamic interaction comprises both adjustment of the cell surface–associated proteome as well as degradation of extracellular matrix (ECM) components. Proteolytic enzymes, and in particular matrix metalloproteinases (MMPs), have emerged as major regulators of these processes, and their central importance for leukocyte migration during extravasation, inflammation, diabetes, and metastasis is widely documented.1,,–4
Membrane-bound MT1-MMP is a “master switch” proteinase in a wide range of cell types. Notably, MT1-MMP is important for key leukocyte functions such as ICAM-1-mediated diapedesis of monocytes,5 migration of monocyte-derived dendritic cells,6 and homing of diabetogenic T cells to pancreatic islets.4 The central role of MT1-MMP in such a variety of processes is reflected by the wide range of substrates that are regulated by its proteolytic activity, comprising extracellular matrix components as well as cell surface–associated and intracellular proteins.3 MT1-MMP cleaves ECM components such as collagen I, II, and III, fibronectin, laminin 1 and 5, and fibrin,7 and adding a further layer of complexity, also proteolytically activates other MMPs such as pro-MMP-2, -MMP-8, and -MMP-13, which in turn are then able to degrade matrix material.3,7 Moreover, MT1-MMP modulates cell adhesion by processing pro-αV and -α5 integrins into their mature forms3 and by promoting the release of ectodomains from several membrane-associated proteins such as CD44, a receptor for hyaluronic acid, laminin, and osteopontin8 or syndecan-1, a receptor for collagens, thrombospondin, and fibronectin.9
To fine-tune MT1-MMP spatiotemporal activity, a complex regulatory network has evolved around this protease. Activation of pro-MT1-MMP by convertases such as furin10 can occur before its insertion into the plasma membrane, and compartmentalization into different lipid domains seems to be important for this process.11 MT1-MMP activity can be inhibited by tissue inhibitors of metalloproteinases (TIMPs), testican,12 or reversion-inducing cysteine-rich protein with Kazal motifs (RECK).7 Finally, degradation of MT1-MMP at the cell surface can be induced by autolysis13 or through MMP-2.14
MT1-MMP regulation also depends on multiple intracellular trafficking events. For example, MT1-MMP has to be delivered to the cell surface, and the GTPase Rab8 has been implicated in this process.15 Surface-exposed MT1-MMP, in turn, can be internalized and recycled, a process that is regulated by both clathrin- or caveolae-dependent pathways.16 Importantly, intracellular trafficking of MT1-MMP has been shown to depend on an intact microtubule system,17 but actual movement of MT1-MMP vesicles on microtubules has not been demonstrated yet.
Podosomes and invadopodia (subsumed under the umbrella term “invadosomes”) are cellular invasion structures that show high local enrichment of MMPs, especially of MT1-MMP, and are thus preferred sites of ECM degradation.18,,–21 The pathways leading to enrichment of MT1-MMP at invadosomes are not well understood, but are likely to involve trafficking of Golgi-derived vesicles.22 A role for microtubules in this process is being discussed,20,23 but has not been confirmed yet.
Here, we present novel data on the intracellular trafficking of MT1-MMP in primary human macrophages. We find that intracellular MT1-MMP is present in Golgi-derived vesicles that move bidirectionally along microtubules. This process is regulated by motor proteins of the kinesin family (KIF5B and KIF3A/KIF3B), as well as dynein. Accordingly, the identified kinesin isoforms are required for the exposure of MT1-MMP on the macrophage surface and for surface-localized activities of MT1-MMP such as shedding of the matrix receptors CD44 and syndecan-1 or for ECM degradation at podosomes. These data show that kinesin-mediated intracellular transport of MT1-MMP is a basal process that allows macrophages to dynamically modify their pericellular environment.
Methods
Cell isolation and cell culture
Human peripheral blood monocytes were isolated from buffy coats and differentiated into macrophages, as described previously.24 In all experiments, cells from at least 3 different donors were evaluated (mostly 30 cells from each donor)
Antibodies and microscopy
Cells were fixed for 10 minutes in 3.7% formaldehyde solution, permeabilized for 4 minutes in ice-cold acetone, and blocked with 2.5% normal human serum and 2.5% normal goat serum (Jackson ImmunoResearch Laboratories) and stained for specific antigens. For details on specific antibodies see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Plasmid constructs and mutagenesis
For detailed information about the generated plasmids, see supplemental Methods. A list of all used kinesin expression constructs used in this study can be found in supplemental Table 2.
Transfection of cells
Cells were transiently transfected using a MicroPorator MP-100 (PeqLab), using the following specifications: pulse voltage 1000 V, pulse width 40 msec, and pulse number 2; and seeded on coverslips at a density of 1 × 105 cells/coverslip.
Live cell imaging
Images were acquired with a spinning disc confocal system. For detailed technical information, see supplemental Methods.
TIRFM
For total interference reflection fluorescence microscopy (TIRFM) experiments, macrophages were analyzed using an Olympus IX-81 inverted microscope equipped with a PLAPON 60×/1.45 NA TIRFM objective. For detailed technical information, see supplemental Methods.
siRNA-induced knockdown
Primary human macrophages were transfected with siRNA (650 ng) twice, at 0 and 48 hours, and evaluated after a further incubation period of 18 hours. For detailed sequences see supplemental Methods.
Flow cytometry
Primary human macrophages were analyzed with a BD FACSCanto II fluorescence activated cell sorter. Data were analyzed with BD FACSDiva software, version 6.0. For detailed procedure and technical information, see supplemental Methods.
ELISA
Commercially available enzyme-linked immunosorbent assays (ELISAs) were used followed by a photometric measurement at a wavelength of 450 nm and of 620 nm as reference. For detailed procedure and technical information, see supplemental Methods.
Immunoblotting
Immunoblotting was performed by standard procedure. For details, see supplemental Methods.
Matrix labeling
Gelatin, fibronectin, or collagen I were fluorescently labeled with NHS-rhodamine according to Chen et al.25 Coverslips were coated with labeled matrix solution, fixed in 0.5% glutaraldehyde. For detailed procedure, see supplemental Methods.
Statistics
Quantifications of anti-mCherry– or other specific antibody-based fluorescence intensities were performed using ImageJ Version 1.42q software (National Institutes of Health). For detailed procedure, see supplemental Methods.
Results
MT1-MMP vesicles travel between a Golgi/MTOC compartment and the cell periphery
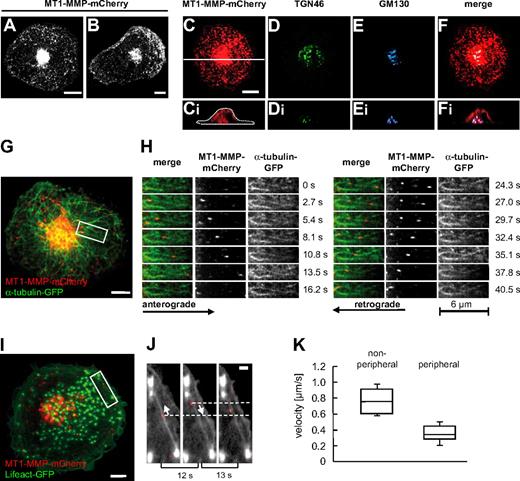
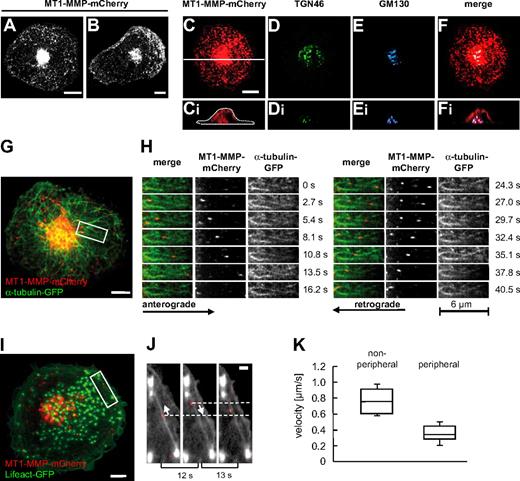
To study the dynamics of MT-MMP in primary human macrophages, cells were transfected with a construct encoding MT1-MMP fused to an intramolecular mCherry tag (see “Transfection of cells”) and analyzed by confocal time-lapse video microscopy. MT1-MMP-mCherry was found to be distributed throughout the cytoplasm in vesicle-like structures with high concentrations in a perinuclear location and in the cell periphery or the leading edge of spontaneously migrating cells (Figure 1A-B). The vesicular nature of these structures was confirmed by colocalization of the green fluorescent protein (GFP)–fused PH domain of phospholipase C-δ1 (PLCδ1-PH-GFP; supplemental Figure 1H), which binds the membrane lipid phosphatidylinositole(4,5)-bisphosphate.26 MT1-MMP-mCherry vesicles were found to originate from the central accumulation and to move into the cell periphery, but centripetal movement was also frequently observed (Figure 1G-H and supplemental Videos 1-2). Costaining or coexpression of a variety of markers for cellular compartments revealed that the central accumulation colocalized especially with Golgi components such as GM130, a cis-Golgi marker, and TGN46, a trans-Golgi protein (Figure 1C-F and supplemental Figure 1A-D), and that it surrounded the microtubule organizing center (MTOC, supplemental Figure 1E-G). Similar colocalizations were detected by staining endogenous MT1-MMP together with TGN46 (supplemental Figure 1A-H) or GM130 (supplemental Figure 1A-H), demonstrating a close match between the localization of overexpressed and endogenous forms of MT1-MMP. Collectively, these data show that MT1-MMP–associated vesicles travel between a central compartment with Golgi/MTOC characteristics and the cell periphery of macrophages.
Localization and dynamics of MT1-MMP-mCherry in macrophages. (A-B) Still images of confocal time-lapse videos (supplemental Videos 1-2) of a macrophage expressing MT1-MMP–mCherry. Note the central accumulation and also vesicles throughout the cytoplasm of a quiescent (A), or at the leading edge of a randomly migrating cell (B). (C-F) Micrographs of a primary macrophage (C) expressing MT1-MMP–mCherry, (D) stained for trans-Golgi marker TGN46 using specific primary and Alexa 488–labeled secondary antibody, and (E) stained for cis-Golgi marker GM130 using specific primary and Cy5-labeled secondary antibody. (F) Merge of images in panels C, D, and E. White bar indicates 10 μm, white line in panel C indicates axis used for the acquisition of respective xz side views shown in panels Ci, Di, Ei, and Fi. (G-H) MT1-MMP–mCherry vesicles move bidirectionally along microtubules. (G) Still image from confocal time-lapse video (supplemental Videos 3-4) of a macrophage expressing MT1-MMP–mCherry and α-tubulin–GFP. White scale bar indicates 10 μm; white box indicates detail area shown in panel H. Detail images (supplemental Video 4) showing movement of MT1-MMP–mCherry vesicles along microtubules. (H left panel) Merged images; (middle panel) MT1-MMP–mCherry signal; (right panel) α-tubulin–GFP signal. Block of images on left shows anterograde movement of a vesicle toward the cell periphery; block of images on right shows retrograde movement of the same vesicle toward the cell center. Time in seconds since the start of the experiment is indicated on the right. (I-J) Movement of MT1-MMP–mCherry vesicles along actin cables in the cell periphery. Confocal micrograph of macrophage expressing MT1-MMP–mCherry and Lifeact-GFP, labeling F-actin. White scale bar indicates 10 μm. White box indicates area shown in detail images in panel J. Detail images from time-lapse video (supplemental Video 5) showing bidirectional movement of MT1-MMP–mCherry along actin cables. Elapsed time between frames is indicated below. White arrows indicate direction of MT1-MMP–mCherry movement. White bar indicates 5 μm. (K) Velocities of MT1-MMP–mCherry vesicles. Box plot diagram shows velocities in micrometers per second for vesicles moving through the central parts of the cell (nonperipheral; n = 20) and for vesicles moving within the cell periphery (within 5 μm of the cell border; peripheral; n = 20). Box plots show mean values with a statistical cut off of ± 25%, with respective SD.
Localization and dynamics of MT1-MMP-mCherry in macrophages. (A-B) Still images of confocal time-lapse videos (supplemental Videos 1-2) of a macrophage expressing MT1-MMP–mCherry. Note the central accumulation and also vesicles throughout the cytoplasm of a quiescent (A), or at the leading edge of a randomly migrating cell (B). (C-F) Micrographs of a primary macrophage (C) expressing MT1-MMP–mCherry, (D) stained for trans-Golgi marker TGN46 using specific primary and Alexa 488–labeled secondary antibody, and (E) stained for cis-Golgi marker GM130 using specific primary and Cy5-labeled secondary antibody. (F) Merge of images in panels C, D, and E. White bar indicates 10 μm, white line in panel C indicates axis used for the acquisition of respective xz side views shown in panels Ci, Di, Ei, and Fi. (G-H) MT1-MMP–mCherry vesicles move bidirectionally along microtubules. (G) Still image from confocal time-lapse video (supplemental Videos 3-4) of a macrophage expressing MT1-MMP–mCherry and α-tubulin–GFP. White scale bar indicates 10 μm; white box indicates detail area shown in panel H. Detail images (supplemental Video 4) showing movement of MT1-MMP–mCherry vesicles along microtubules. (H left panel) Merged images; (middle panel) MT1-MMP–mCherry signal; (right panel) α-tubulin–GFP signal. Block of images on left shows anterograde movement of a vesicle toward the cell periphery; block of images on right shows retrograde movement of the same vesicle toward the cell center. Time in seconds since the start of the experiment is indicated on the right. (I-J) Movement of MT1-MMP–mCherry vesicles along actin cables in the cell periphery. Confocal micrograph of macrophage expressing MT1-MMP–mCherry and Lifeact-GFP, labeling F-actin. White scale bar indicates 10 μm. White box indicates area shown in detail images in panel J. Detail images from time-lapse video (supplemental Video 5) showing bidirectional movement of MT1-MMP–mCherry along actin cables. Elapsed time between frames is indicated below. White arrows indicate direction of MT1-MMP–mCherry movement. White bar indicates 5 μm. (K) Velocities of MT1-MMP–mCherry vesicles. Box plot diagram shows velocities in micrometers per second for vesicles moving through the central parts of the cell (nonperipheral; n = 20) and for vesicles moving within the cell periphery (within 5 μm of the cell border; peripheral; n = 20). Box plots show mean values with a statistical cut off of ± 25%, with respective SD.
MT1-MMP vesicles move bidirectionally along microtubules and actin filaments
The microtubule-dependent distribution of MT1-MMP-mCherry vesicles suggested an association with the microtubule system, as observed earlier in MCF7 breast carcinoma cells.17 Indeed, immunofluorescence analysis showed that MT1-MMP–mCherry accumulations were in most cases in close contact with microtubules (supplemental Figure 1I). Again, this finding was confirmed by detection of endogenous MT1-MMP (supplemental Figure 1Ji-iii). Furthermore, confocal time-lapse analysis of cells coexpressing GFP-labeled α-tubulin confirmed that MT1-MMP-mCherry vesicles travel along microtubules (Figure 1G-H and supplemental Videos 3-4). This movement was bidirectional (Figure 1G-H), with observed velocities in the same range (0.78 ± 0.2 μm/s; n = 20) for both anterograde and retrograde direction (Figure 1K). As MT1-MMP-mCherry vesicles were also often observed to switch direction spontaneously (Figure 1H), this suggested an association with both microtubule plus end– and minus end–directed motors.
In addition to this bidirectional long-range transport, MT1-MMP–mCherry vesicles were also found to move within the cell periphery. This short range transport involved movement of MT1-MMP-mCherry along actin cables (Figure 1I-J and supplemental Video 5) and proceeded with markedly lower velocities (0.2-0.5 μm/s; n = 17; Figure 1K).
Microtubule-based retrograde transport of MT1-MMP depends on dynein
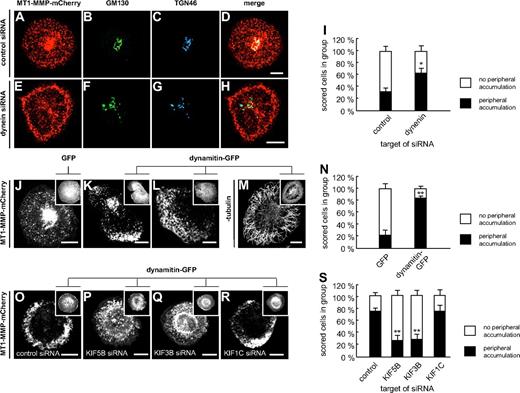
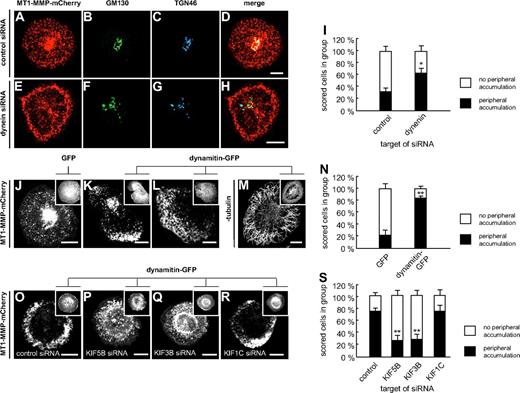
To study the microtubule-based transport of MT1-MMP in more detail, we first investigated the potential involvement of minus-end directed dynein. This was addressed by treating cells with siRNA specific for dynein27 or for luciferase, as a control (Figure 2A-I). SiRNA-induced knockdown of dynein resulted in increased numbers of cells showing accumulations of MT1-MMP–mCherry vesicles in the cell periphery (Figure 2E), compared with controls (Figure 2I), as well as a more dispersed appearance of the Golgi (Figure 2F-G), as described earlier.28 This observation was also made when endogenous MT1-MMP was stained in dynein siRNA-treated cells (data not shown).
Transport of MT1-MMP along microtubules is driven by dynein and kinesins. (A-I) Dynein siRNA leads to peripheral accumulation of MT1-MMP and to Golgi dispersal. Confocal micrographs of primary human macrophages expressing MT1-MMP–mCherry (A,E), transfected with control siRNA (A-D) or dynein siRNA (E-H), and stained for Golgi markers GM130 (B,F) and TGN46 (C,G). (D,H) Merged images. Note peripheral accumulation of MT1-MMP–mCherry (E) and dispersed appearance of Golgi (F-G) upon dynein siRNA treatment. (I) Evaluation of MT1-MMP–mCherry distribution upon transfection with control siRNA or siRNA against dynein. For each value, 3 × 30 cells were evaluated. Note enhanced percentage of cells with peripheral accumulation of MT1-MMP–mCherry in case of dynein siRNA treatment. Asterisk indicates value significantly different from controls (P < .05). For specific values, see supplemental Table 1. (J-N) Retrograde movement of MT1-MMP depends on dynein. Still images of confocal time-lapse videos of macrophages expressing MT1-MMP–mCherry and coexpressing GFP (J) or dynamitin-GFP for inhibition of dynein (K-L; supplemental Video 6), as indicated in detail images. Note pronounced peripheral accumulation of MT1-MMP–mCherry upon dynein inhibition in (K-L), mostly accompanied by reduction (K) or disappearance (L) of the central accumulation (supplemental Video 6). (M) Confocal micrograph of macrophage expressing dynamitin-GFP and stained for β-tubulin by specific primary and Alexa 488–labeled secondary antibody. Note intact microtubule system in case of dynamitin-GFP overexpression. (N) Evaluation of intracellular MT1-MMP–mCherry distribution upon overexpression of dynamitin-GFP or GFP control. For each value, 3 × 30 cells were evaluated. Note increased percentage of cells with peripheral accumulation of MT1-MMP–mCherry upon overexpression of dynamitin-GFP. **P < .001 compared with control. (O-S) Anterograde movement of MT1-MMP depends on KIF5B and KIF3B kinesins. Confocal images of macrophages expressing MT1-MMP–mCherry and coexpressing dynamitin-GFP, as indicated in detail images. Cells were treated with siRNA against control sequence (O), KIF5B (P), KIF3B (Q), or KIF1C (R). Note pronounced peripheral accumulation of MT1-MMP–mCherry upon dynein inhibition in control cells treated with control siRNA (O). This effect is inhibited by treatment with siRNA directed against KIF5B (P), KIF3B (Q), but not KIF1C (R). (S) Evaluation of cells showing peripheral accumulation of MT1-MMP–mCherry upon overexpression of dynamitin-GFP and treatment with various siRNAs. For each value, 3 × 30 cells were evaluated. **P < .001 compared with control. White scale bars indicate 10 μm.
Transport of MT1-MMP along microtubules is driven by dynein and kinesins. (A-I) Dynein siRNA leads to peripheral accumulation of MT1-MMP and to Golgi dispersal. Confocal micrographs of primary human macrophages expressing MT1-MMP–mCherry (A,E), transfected with control siRNA (A-D) or dynein siRNA (E-H), and stained for Golgi markers GM130 (B,F) and TGN46 (C,G). (D,H) Merged images. Note peripheral accumulation of MT1-MMP–mCherry (E) and dispersed appearance of Golgi (F-G) upon dynein siRNA treatment. (I) Evaluation of MT1-MMP–mCherry distribution upon transfection with control siRNA or siRNA against dynein. For each value, 3 × 30 cells were evaluated. Note enhanced percentage of cells with peripheral accumulation of MT1-MMP–mCherry in case of dynein siRNA treatment. Asterisk indicates value significantly different from controls (P < .05). For specific values, see supplemental Table 1. (J-N) Retrograde movement of MT1-MMP depends on dynein. Still images of confocal time-lapse videos of macrophages expressing MT1-MMP–mCherry and coexpressing GFP (J) or dynamitin-GFP for inhibition of dynein (K-L; supplemental Video 6), as indicated in detail images. Note pronounced peripheral accumulation of MT1-MMP–mCherry upon dynein inhibition in (K-L), mostly accompanied by reduction (K) or disappearance (L) of the central accumulation (supplemental Video 6). (M) Confocal micrograph of macrophage expressing dynamitin-GFP and stained for β-tubulin by specific primary and Alexa 488–labeled secondary antibody. Note intact microtubule system in case of dynamitin-GFP overexpression. (N) Evaluation of intracellular MT1-MMP–mCherry distribution upon overexpression of dynamitin-GFP or GFP control. For each value, 3 × 30 cells were evaluated. Note increased percentage of cells with peripheral accumulation of MT1-MMP–mCherry upon overexpression of dynamitin-GFP. **P < .001 compared with control. (O-S) Anterograde movement of MT1-MMP depends on KIF5B and KIF3B kinesins. Confocal images of macrophages expressing MT1-MMP–mCherry and coexpressing dynamitin-GFP, as indicated in detail images. Cells were treated with siRNA against control sequence (O), KIF5B (P), KIF3B (Q), or KIF1C (R). Note pronounced peripheral accumulation of MT1-MMP–mCherry upon dynein inhibition in control cells treated with control siRNA (O). This effect is inhibited by treatment with siRNA directed against KIF5B (P), KIF3B (Q), but not KIF1C (R). (S) Evaluation of cells showing peripheral accumulation of MT1-MMP–mCherry upon overexpression of dynamitin-GFP and treatment with various siRNAs. For each value, 3 × 30 cells were evaluated. **P < .001 compared with control. White scale bars indicate 10 μm.
However, knockdown of dynein (over 6 days) resulted in a large number of detaching, nonviable cells, potentially influencing the results. Therefore, we also inhibited dynein function by overnight expression of GFP-fused dynamitin, which disrupts transport and localization of dynein-dependent organelles.29 Coexpression of dynamitin-GFP resulted in an accumulation of MT1-MMP–mCherry vesicles in the cell periphery (Figure 2J-N), which was often accompanied by a reduction of the central accumulation, compatible with earlier observations of dynamitin overexpression also influencing Golgi integrity.29 Concomitantly, retrograde movement of MT1-MMP-mCherry vesicles ceased in most cases (Figure 2K,L,N, supplemental Table 1, and supplemental Video 6). Similar results were gained by inhibition of dynein using the chemical inhibitor erythro-9-[3-(2-hydroxynonyl)] adenine (EHNA; supplemental Figure 2 and supplemental Table 1).30 In all cases, the integrity of the microtubule system was not compromised (Figure 2M and not shown). Combined, these results indicate that retrograde movement of MT1-MMP–mCherry vesicles along microtubules depends on cytoplasmic dynein.
KIF5B and KIF3A/KIF3B kinesins regulate anterograde transport of MT1-MMP
To investigate the involvement of microtubule-associated motors in anterograde movement of MT1-MMP vesicles, GFP- or yellow fluorescent protein (YFP)–fused constructs of wt kinesin isoforms were analyzed for colocalization with these vesicles. Partial colocalization was observed for KIF5B (supplemental Figure 3A-C), a member of the kinesin-1 family, and for KIF3B and KIF3A (supplemental Figure 3D-F and not shown), subunits of heterotrimeric kinesin, a kinesin-2 family member, in both fixed and living specimens, but not for other kinesins such as KIF1C or Kif5c or KIF9 (supplemental Figure 3G-L and not shown). Staining of endogenous MT1-MMP in cells overexpressing various kinesin constructs (supplemental Figure 4) and, vice versa, staining of endogenous KIF5B or KIF3A in cells overexpressing MT1-MMP–mCherry (supplemental Figure 5) confirmed a specific colocalization for both KIF5B and KIF3A/KIF3B with MT1-MMP.
To get direct proof that these kinesins are involved in the transport of MT1-MMP vesicles, this analysis was followed by knockdown of kinesin isoforms using validated siRNAs (see Methods).24 However, disturbance of kinesin activity by knockdown of individual kinesins led to very diverse phenotypes of MT1-MMP distribution, which are hard to characterize. Still, these experiments gave important hints that KIF5B and KIF3A/KIF3B knock down leads to aberrant distribution of both MT1-MMP–mCherry (supplemental Figure 6A-E) and endogenous MT1-MMP (supplemental Figure 6F-J).
In a next step, we made use of the observation that inhibition of retrograde movement through overexpression of dynamitin leads to enrichment of MT1-MMP vesicles in the cell periphery (Figure 2J-N). This accumulation depends on the (presumably kinesin-driven) delivery of MT1-MMP to the cell periphery and serves as a very clear readout of aberrant MT1-MMP distribution. This phenotype was preserved in control cells cotransfected with a GFP-dynamitin construct and siRNA against firefly luciferase (Figure 2O,S) and also in cells cotransfected with KIF1C-specific siRNA (Figure 2R-S). By contrast, cotransfection with siRNAs directed against KIF5B (Figure 2P,S) or KIF3B (Figure 2Q,S) did not lead to peripheral enrichment of MT1-MMP vesicles (see also supplemental Table 1). Combined, these results suggest that anterograde movement of MT1-MMP–mCherry vesicles toward the cell periphery depends on both KIF5B and KIF3A/KIF3B.
Cell-surface exposure of MT1-MMP is mediated by KIF5B and KIF3A/KIF3B
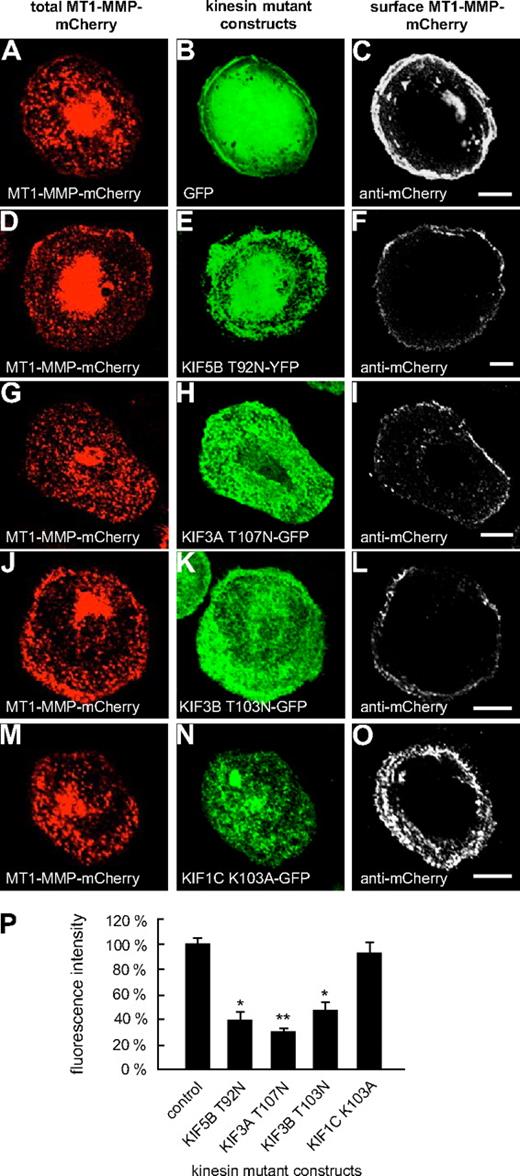
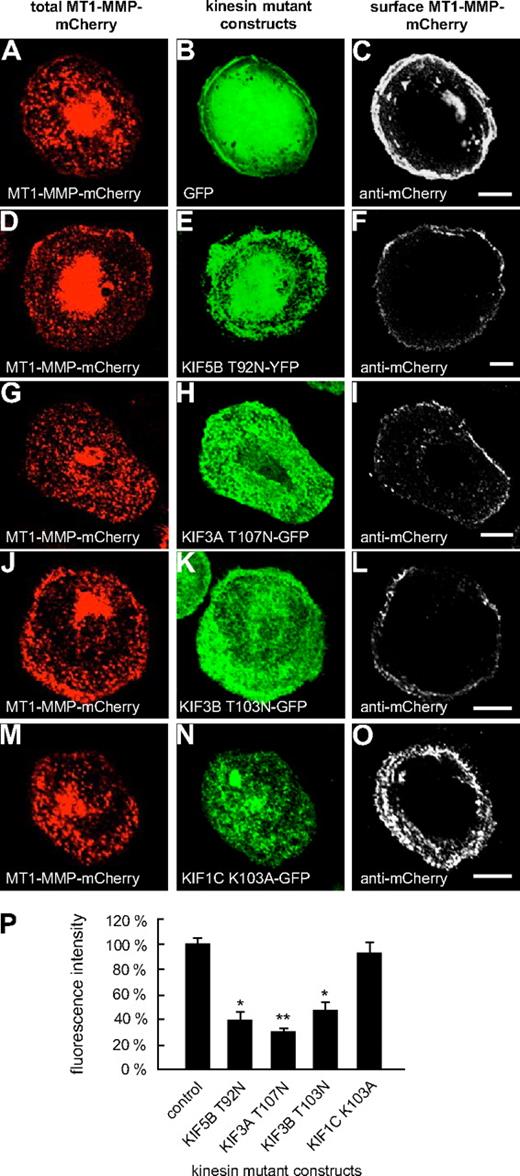
Typically, kinesins feature a P-loop sequence in the N-terminal region, which is involved in nucleotide binding.31 Mutations within this sequence (GX4GKS/T) have been shown to block ATP hydrolysis and to induce a rigor state in KIF5B (T92N)32 or to lead to dysfunction of KIF1C (K103A).24 Therefore, respective GFP- or YFP-fused mutant constructs (KIF3A-T107N; KIF3B-T103N, KIF5B-T92N; KIF1C-K103A; supplemental Table 2) were generated and evaluated for their effects on the cell-surface exposure of MT1-MMP–mCherry. Eighteen hours after transfection, cells were fixed, but not permeabilized, and stained with a polyclonal anti-mCherry antibody (see “Antibodies and microscopy”) and a secondary Cy5-labeled antibody to visualize surface exposed MT1-MMP–mCherry (Figure 3). Confocal laser scanning analysis performed with identical laser intensities showed that compared with control cells coexpressing GFP, surface-accessible MT1-MMP–mCherry was clearly reduced upon coexpression of KIF5B-T92N (Figure 3D-F,P and supplemental Figure 7), KIF3A-T107N (Figure 3G-I,P and supplemental Figure 7), and KIF3B-T103N (Figure 3J-L,P and supplemental Figure 7), but not of KIF1C-K103A (Figure 3M-P and supplemental Figure 7), or of respective wild-type constructs (supplemental Figure 8).
Overexpression of rigor constructs of KIF5B, KIF3A, and KIF3B influences cell-surface exposure of MT1-MMP. Confocal micrographs of macrophages expressing MT1-MMP–mCherry transfected with (A-C) GFP control or GFP- or YFP-fused P-loop mutant constructs of kinesins: (D-F) KIF5B T92N, (G-I) KIF3A T107N, (J-L) KIF3B T103N, and (M-O) KIF1C K103A. Total MT1-MMP–mCherry signal shown in (A,D,G,J,M; red), GFP signal in (B,E,H,K,N; green). Cells were fixed, but not permeabilized, and stained with primary anti-mCherry antibody and secondary Cy5-conjugated antibody to label MT1-MMP–mCherry on the cell surface (C,F,I,L,O; white). Note reduction of MT1-MMP–mCherry at the cell surface upon overexpression of KIF5B, KIF3A, and KIF3B mutants, but not in case of the KIF1C mutant. White bar indicates 10 μm for all images of the same row. (P) Fluorescence intensities of surface-localized MT1-MMP–mCherry, based on Cy5 fluorescence. Bars indicate mean values ± SD. Fluorescence intensity for the control (A-C) was set to 100%. *P < .05, **P < .01 compared with control. For all values, 3 × 30 cells were evaluated. For specific values, see supplemental Table 1.
Overexpression of rigor constructs of KIF5B, KIF3A, and KIF3B influences cell-surface exposure of MT1-MMP. Confocal micrographs of macrophages expressing MT1-MMP–mCherry transfected with (A-C) GFP control or GFP- or YFP-fused P-loop mutant constructs of kinesins: (D-F) KIF5B T92N, (G-I) KIF3A T107N, (J-L) KIF3B T103N, and (M-O) KIF1C K103A. Total MT1-MMP–mCherry signal shown in (A,D,G,J,M; red), GFP signal in (B,E,H,K,N; green). Cells were fixed, but not permeabilized, and stained with primary anti-mCherry antibody and secondary Cy5-conjugated antibody to label MT1-MMP–mCherry on the cell surface (C,F,I,L,O; white). Note reduction of MT1-MMP–mCherry at the cell surface upon overexpression of KIF5B, KIF3A, and KIF3B mutants, but not in case of the KIF1C mutant. White bar indicates 10 μm for all images of the same row. (P) Fluorescence intensities of surface-localized MT1-MMP–mCherry, based on Cy5 fluorescence. Bars indicate mean values ± SD. Fluorescence intensity for the control (A-C) was set to 100%. *P < .05, **P < .01 compared with control. For all values, 3 × 30 cells were evaluated. For specific values, see supplemental Table 1.
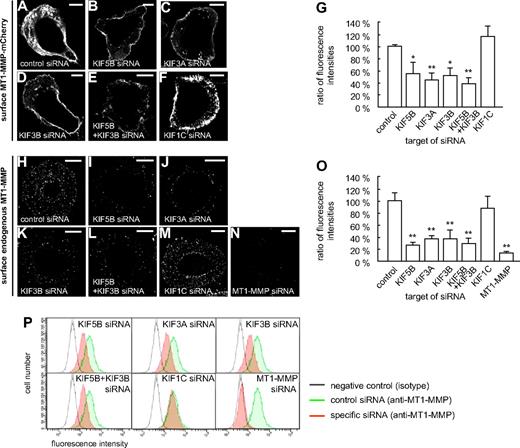
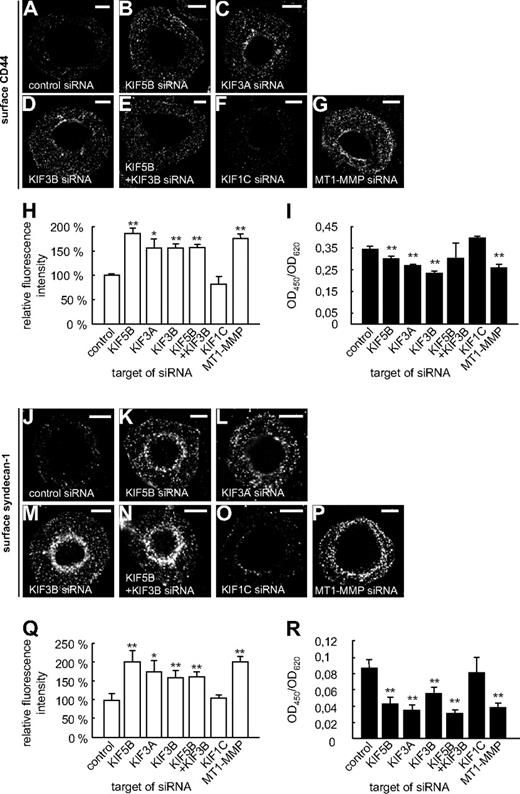
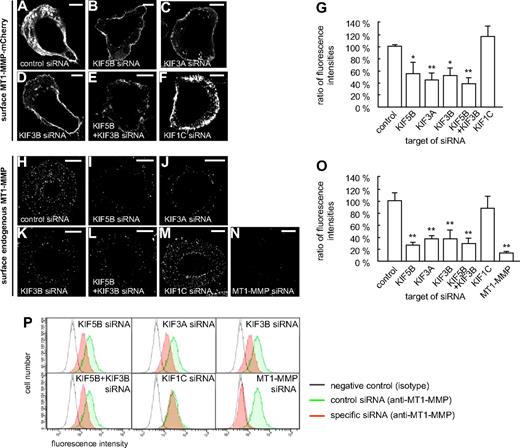
These experiments suggested that both KIF5B and KIF3A/KIF3B play a role in the delivery of MT1-MMP to the cell surface. To get direct proof, macrophages were cotransfected with the MT1-MMP–mCherry construct and siRNAs specific for KIF5B, KIF3A, and KIF3B, as well as for KIF1C and a control sequence. After 3 days, cells were fixed, but not permeabilized, and stained with the anti-mCherry antibody and a Cy5-labeled secondary antibody to visualize surface-accessible MT1-MMP–mCherry (Figure 4A-G). For each condition, total cellular MT1-MMP–mCherry, based on mCherry fluorescence intensities, and surface-localized MT1-MMP–mCherry, based on Cy5 fluorescence intensities, were measured for 3 times 30 cells (supplemental Figure 9 and supplemental Table 1). The respective ratios were calculated, and the mean ratio for cells treated with control siRNA was set to 100% (Figure 4G). In line with our earlier findings, relative levels of MT1-MMP–mCherry on the cell surface were significantly decreased upon treatment with siRNAs specific for KIF5B, KIF3A, and KIF3B, but not for KIF1C or the control siRNA (Figure 4G and supplemental Table 1). Compared with knockdowns of single isoforms, double knockdown of KIF5B and KIF3B resulted in a slightly more pronounced, although not significantly different, effect (Figure 4G). In all cases, average cell sizes were found to be comparable (not shown). In addition, we also performed kinesin knockdown experiments and stained cells for endogenous, surface-localized MT1-MMP (Figure 4H-M), with knockdown of endogenous MT1-MMP (supplemental Figure 10) as a negative control (Figure 4N). These experiments confirmed the data gained with cells overexpressing MT1-MMP–mCherry.
Cell-surface exposure of MT1-MMP is mediated by KIF5B and KIF3A/KIF3B, but not by KIF1C. (A-F) Confocal micrographs of macrophages overexpressing MT1-MMP–mCherry (A-F) and wildtype macrophages (H-N), each treated with siRNA against (A,H) control sequence, (B,I) KIF5B, (C,J) KIF3A, (D,K) KIF3B, (E,L) KIF5B and KIF3B, (F,M) KIF1C, or (N) MT1-MMP. Cells were fixed, but not permeabilized, and stained with primary anti-mCherry (A-F) or anti–MT1-MMP (H-N) antibody, respectively, and secondary Cy5-conjugated antibody to label MT1-MMP–mCherry (A-F) or endogenous MT1-MMP (H-N) on the cell surface. White scale bar indicates 10 μm for all images. (G) Ratio of surface MT1-MMP–mCherry based on Cy5 fluorescence versus total cellular MT1-MMP–mCherry based on mCherry fluorescence. Fluorescence intensities for the control siRNA were set to 100%. *P < .05, **P < .01 compared with control. Bars indicate mean values ± SD. (O) Fluorescence intensities of surface-localized endogenous MT1-MMP, based on Cy5 fluorescence. Fluorescence intensities for the control siRNA were set to 100%. **P < .01 compared with control. For all values, 3 × 30 cells were evaluated. For specific values, see supplemental Table 1. Bars indicate mean values ± SD. (P) Flow cytometric analysis of endogenous MT1-MMP on the cell surface. Macrophages were treated with siRNA against control sequence, KIF5B, KIF3A, KIF3B, KIF5B and KIF3B, KIF1C, or MT1-MMP and stained for surface MT1-MMP and Alexa 488–labeled secondary antibody without fixation. Cells treated with control siRNA and stained with isotype IgG antibody were used as a negative control (gray). Note lower fluorescence intensity of cells transfected with siRNA against KIF5B, KIF3A, or KIF3B, compared with siRNA against KIF1C or unspecific control siRNA (green). Results from 1 of 2 respective experiments are shown. For specific values, see supplemental Table 1.
Cell-surface exposure of MT1-MMP is mediated by KIF5B and KIF3A/KIF3B, but not by KIF1C. (A-F) Confocal micrographs of macrophages overexpressing MT1-MMP–mCherry (A-F) and wildtype macrophages (H-N), each treated with siRNA against (A,H) control sequence, (B,I) KIF5B, (C,J) KIF3A, (D,K) KIF3B, (E,L) KIF5B and KIF3B, (F,M) KIF1C, or (N) MT1-MMP. Cells were fixed, but not permeabilized, and stained with primary anti-mCherry (A-F) or anti–MT1-MMP (H-N) antibody, respectively, and secondary Cy5-conjugated antibody to label MT1-MMP–mCherry (A-F) or endogenous MT1-MMP (H-N) on the cell surface. White scale bar indicates 10 μm for all images. (G) Ratio of surface MT1-MMP–mCherry based on Cy5 fluorescence versus total cellular MT1-MMP–mCherry based on mCherry fluorescence. Fluorescence intensities for the control siRNA were set to 100%. *P < .05, **P < .01 compared with control. Bars indicate mean values ± SD. (O) Fluorescence intensities of surface-localized endogenous MT1-MMP, based on Cy5 fluorescence. Fluorescence intensities for the control siRNA were set to 100%. **P < .01 compared with control. For all values, 3 × 30 cells were evaluated. For specific values, see supplemental Table 1. Bars indicate mean values ± SD. (P) Flow cytometric analysis of endogenous MT1-MMP on the cell surface. Macrophages were treated with siRNA against control sequence, KIF5B, KIF3A, KIF3B, KIF5B and KIF3B, KIF1C, or MT1-MMP and stained for surface MT1-MMP and Alexa 488–labeled secondary antibody without fixation. Cells treated with control siRNA and stained with isotype IgG antibody were used as a negative control (gray). Note lower fluorescence intensity of cells transfected with siRNA against KIF5B, KIF3A, or KIF3B, compared with siRNA against KIF1C or unspecific control siRNA (green). Results from 1 of 2 respective experiments are shown. For specific values, see supplemental Table 1.
Similar results were gained by flow cytometric analysis of cells treated with kinesin-specific or MT1-MMP–specific siRNA (Figure 4P and supplemental Table 1), which indicated that knock down of KIF5B, KIF3A, or KIF3B, but not of KIF1C, led to a reduced surface exposure of endogenous MT1-MMP. Collectively, these experiments indicate that transport by KIF5B and KIF3A/KIF3B kinesins, but not by KIF1C, is necessary for efficient exposure of MT1-MMP on the cell surface.
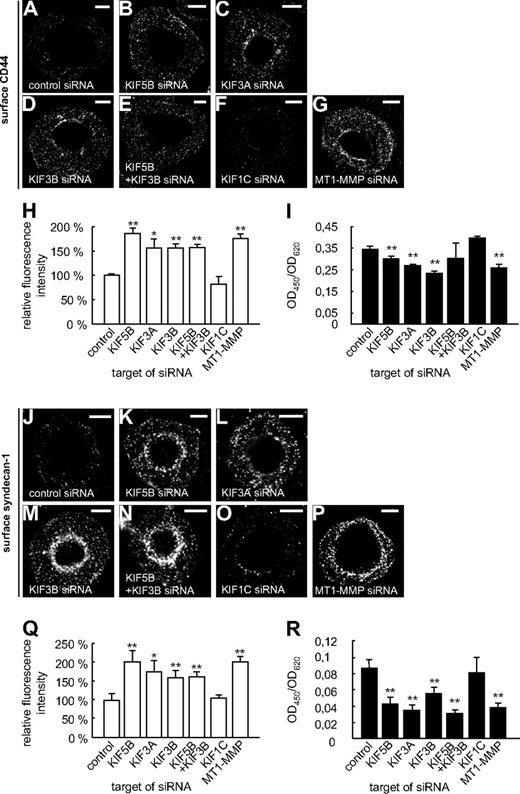
KIF5B and KIF3A/KIF3B regulate shedding of CD44 and syndecan-1
Surface-associated MT1-MMP is important for the shedding (ie, the proteolytic release of ectodomains) of the matrix receptors CD448 and syndecan-1.9 Therefore, we next investigated potential functional consequences of decreased surface levels of MT1-MMP under conditions of kinesin knockdown. For this, macrophages were transfected with kinesin- or MT1-MMP–specific siRNAs. After 3 days, cells were fixed, but not permeabilized, and CD44 or syndecan-1 on the cell surface was stained using specific antibodies recognizing epitopes within the respective ectodomains, as well as Cy5-labeled secondary antibodies (Figure 5A-H,J-Q). The level of surface-localized ectodomains, based on Cy5-fluorescence intensities, was measured for 3 times 30 cells. The respective ratios were calculated, and the mean ratio for cells treated with luciferase siRNA was set to 100%. For both CD44 (Figure 5H) and syndecan-1 (Figure 5Q), relative levels of ectodomains present on the cell surface were significantly increased in cells treated with siRNAs specific for KIF5B, KIF3A, KIF3B, and also for MT1-MMP or a double knockdown of KIF5B and KIF3B, but not for KIF1C (Figure 5F,H and supplemental Table 1). To complement these experiments, we also used enzyme-linked immunosorbent assays to detect the shedded material in supernatants of the cells used in knock down experiments. Indeed, knockdown of KIF5B, KIF3A, or KIF3B, but not of KIF1C, led to reduced levels of shedded CD44- (Figure 5I and supplemental Table 1) or syndecan-1 (Figure 5R and supplemental Table 1) ectodomains in the culture supernatants. Combined, these experiments show that knockdown of specific kinesins, resulting in reduced surface levels of MT1-MMP, also leads to impairment of MT1-MMP–based activities at the dorsal cell surface.
Shedding of CD44 or syndecan-1 is mediated by KIF5B and KIF3A/KIF3B, but not by KIF1C. (A-G,J-P) Confocal micrographs of macrophages transfected with siRNA against (A,J) control sequence, (B,K) KIF5B, (C,L) KIF3A, (D,M) KIF3B, (E,N) KIF5B and KIF3B, (F,O) KIF1C, or (G,P) MT1-MMP. Cells were fixed, but not permeabilized, and stained with primary anti-CD44 (A-G) or anti–syndecan-1 (J-P) antibody, respectively, recognizing an epitope in the ectodomain, and secondary Cy5-conjugated antibody. White scale bar indicates 10 μm for all images. (H,Q) Fluorescence intensities of surface-localized (H) CD44 or (Q) syndecan-1, based on Cy5 fluorescence. Fluorescence intensities for the control siRNA were set to 100%. For all values, 3 × 30 cells were evaluated. For specific values, see supplemental Table 1. (I,R) ELISA for measuring shedded ectodomains of (I) CD44 or (R) syndecan-1 in culture media of siRNA-treated cells described in panels A through H and J through Q. Data were evaluated using the ratio of OD450 to OD620 to exclude a noncompleted development of the substrate. Experiments were performed twice in triplicates, with values shown as mean ± SD of 1 experiment. For specific values, see supplemental Table 1. *P < .05, **P < .01 compared with control.
Shedding of CD44 or syndecan-1 is mediated by KIF5B and KIF3A/KIF3B, but not by KIF1C. (A-G,J-P) Confocal micrographs of macrophages transfected with siRNA against (A,J) control sequence, (B,K) KIF5B, (C,L) KIF3A, (D,M) KIF3B, (E,N) KIF5B and KIF3B, (F,O) KIF1C, or (G,P) MT1-MMP. Cells were fixed, but not permeabilized, and stained with primary anti-CD44 (A-G) or anti–syndecan-1 (J-P) antibody, respectively, recognizing an epitope in the ectodomain, and secondary Cy5-conjugated antibody. White scale bar indicates 10 μm for all images. (H,Q) Fluorescence intensities of surface-localized (H) CD44 or (Q) syndecan-1, based on Cy5 fluorescence. Fluorescence intensities for the control siRNA were set to 100%. For all values, 3 × 30 cells were evaluated. For specific values, see supplemental Table 1. (I,R) ELISA for measuring shedded ectodomains of (I) CD44 or (R) syndecan-1 in culture media of siRNA-treated cells described in panels A through H and J through Q. Data were evaluated using the ratio of OD450 to OD620 to exclude a noncompleted development of the substrate. Experiments were performed twice in triplicates, with values shown as mean ± SD of 1 experiment. For specific values, see supplemental Table 1. *P < .05, **P < .01 compared with control.
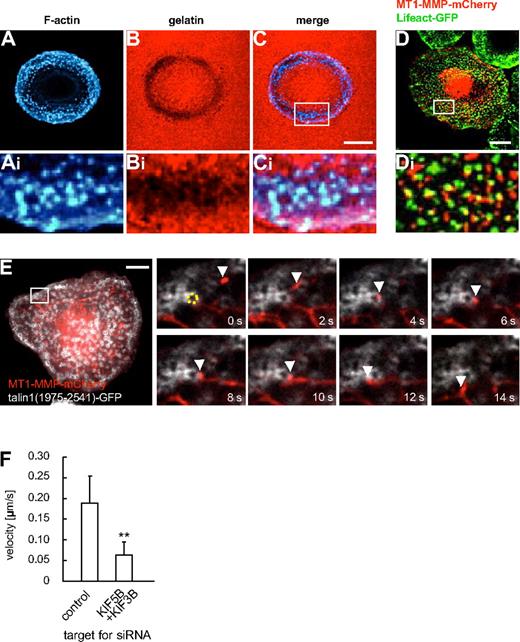
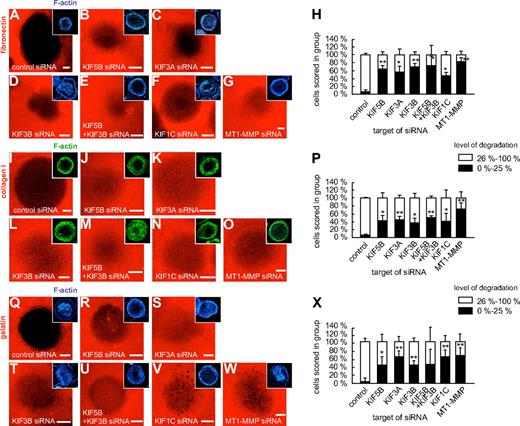
Matrix degradation at podosomes is regulated by KIF5B, KIF3A/KIF3B, and KIF1C
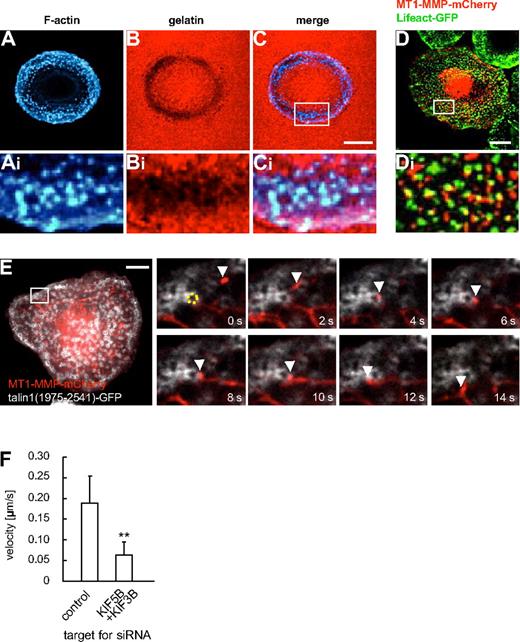
Podosomes, actin-rich structures involved in ECM degradation, are prominent examples of MT1-MMP activity at the ventral surface of cells.20,23 In primary macrophages, podosomes are the predominant sites of matrix degradation, which can be demonstrated by seeding cells on fluorescently labeled matrix (Figure 6A-C). The pathways leading to enrichment of MT1-MMP at podosomes are not well understood,20,23 although at invadopodia, podosome-related structures, they involve the machinery for vesicle docking and fusion, such as the v-SNARE VAMP733 or the exocyst complex,34 Coexpression of MT1-MMP–mCherry and GFP-coupled Lifeact,35 staining F-actin rich podosome cores, revealed that MT1-MMP–mCherry vesicles were often in close proximity to podosomes (Figure 6D). This was confirmed by confocal time-lapse microscopy, where MT1-MMP–mCherry vesicles were found to dynamically and repeatedly contact the F-actin–rich core of podosomes (data not shown). To study this in more detail, we next labeled the podosome ring structure, which surrounds the actin core,20 by expression of a GFP-fused construct of talin1 (comprising amino acid residues 1975-2541).36 Confocal time-lapse analysis showed that MT1-MMP vesicles often colocalized with podosomes rings and also appeared to move along them (Figure 6E and supplemental Video 7). Contact of vesicles with podosome rings was often accompanied by a significant deceleration of the vesicles (eg, contact duration 10 seconds, with a travel distance of approximately 0.5 μm in Figure 6E), compared with the speed of MT1-MMP vesicles in other regions of the cell (see Figure 1K). The same phenomenon could be observed using TIRFM (supplemental Figure 11). Interestingly, double knockdown of KIF5B and KIF3B led to a significantly lower velocity of MT1-MMP vesicles in the vicinity of podosomes, indicating an influence of kinesin motor activity (Figure 6I and supplemental Table 1). Combined, these observations suggest a close, comparably long-lasting and dynamic contact between MT1-MMP vesicles and podosome substructures within a zone near the plane of matrix contact.
Podosomes are sites of matrix degradation and are contacted by MT1-MMP–mCherry vesicles. (A-F) Confocal micrographs of a macrophage seeded on rhodamine-labeled gelatin matrix, F-actin labeling podosome cores stained with Cy5-labeled phalloidin (A,Ai), gelatin matrix in red (B,Bi), merged images (C,Ci). White box in (C) indicates region enlarged in (Ai,Bi,Ci). (D) Confocal micrograph of primary human macrophage expressing MT1-MMP–mCherry and GFP-coupled Lifeact, staining F-actin-rich podosome cores. White box in (D) indicates region shown enlarged in (Di). Note close proximity between MT1-MMP–mCherry vesicles and podosome cores. (E) Still images from time-lapse video (supplemental Video 7). Macrophage expressing MT1-MMP–mCherry and a C-terminal talin1-GFP construct labeling podosome ring structures. White box in larger image indicates region shown in detail images. A podosome (labeled by dotted yellow ring) is contacted by a MT1-MMP-mCherry vesicle (white arrowhead) for approximately 10 seconds. Time in seconds is indicated in lower right corners. White scale bars indicate 10 μm. (F) Velocity of MT1-MMP–mCherry vesicles in the vicinity of podosomes. Bar diagram shows velocities in micrometers per second for vesicles moving along podosome rings in macrophages treated with control siRNA or 2 siRNAs specific for KIF5B and KIF3B (n = 17, respectively). **P < .01 compared with control. For specific values, see supplemental Table 1. Bars indicate mean values ± SD.
Podosomes are sites of matrix degradation and are contacted by MT1-MMP–mCherry vesicles. (A-F) Confocal micrographs of a macrophage seeded on rhodamine-labeled gelatin matrix, F-actin labeling podosome cores stained with Cy5-labeled phalloidin (A,Ai), gelatin matrix in red (B,Bi), merged images (C,Ci). White box in (C) indicates region enlarged in (Ai,Bi,Ci). (D) Confocal micrograph of primary human macrophage expressing MT1-MMP–mCherry and GFP-coupled Lifeact, staining F-actin-rich podosome cores. White box in (D) indicates region shown enlarged in (Di). Note close proximity between MT1-MMP–mCherry vesicles and podosome cores. (E) Still images from time-lapse video (supplemental Video 7). Macrophage expressing MT1-MMP–mCherry and a C-terminal talin1-GFP construct labeling podosome ring structures. White box in larger image indicates region shown in detail images. A podosome (labeled by dotted yellow ring) is contacted by a MT1-MMP-mCherry vesicle (white arrowhead) for approximately 10 seconds. Time in seconds is indicated in lower right corners. White scale bars indicate 10 μm. (F) Velocity of MT1-MMP–mCherry vesicles in the vicinity of podosomes. Bar diagram shows velocities in micrometers per second for vesicles moving along podosome rings in macrophages treated with control siRNA or 2 siRNAs specific for KIF5B and KIF3B (n = 17, respectively). **P < .01 compared with control. For specific values, see supplemental Table 1. Bars indicate mean values ± SD.
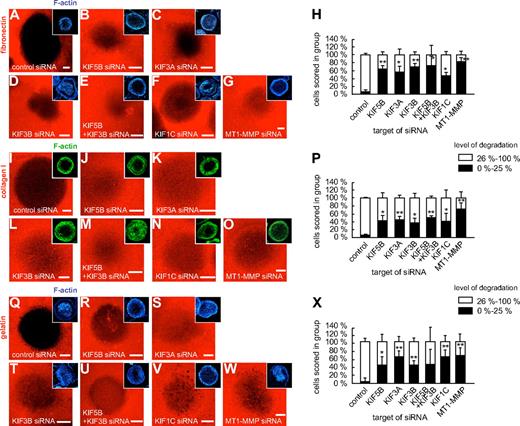
As MT1-MMP travels along microtubules, which contact podosomes by their plus ends,24 we next investigated the potential impact of plus end-directed kinesins on MT1-MMP–dependent matrix degradation at podosomes. For this, macrophages were transfected with siRNAs specific for KIF5B, KIF3A, KIF3B, and KIF1C, as well as for luciferase and MT1-MMP. After 3 days, cells were reseeded on fibronectin matrix fluorescently labeled with rhodamine, and matrix degradation was evaluated after 6 hours (Figure 7). Matrix degradation was analyzed by measuring the rhodamine-based fluorescence of the podosome-covered area, and cells (n = 3 × 30) were scored into groups according to the degree of matrix degradation (0%-25%; 26%-100%; Figure 7H,P,X). The number of cells showing low (0%-25%) matrix degradation was significantly enhanced in cells transfected with siRNA specific for KIF5B, KIF3A, KIF3B, KIF3A/KIF3B, KIF1C, and also for MT1-MMP (Figure 7H,P,X and supplemental Table 1). Similar results were obtained with collagen I as a substratum (Figure 7I-P and supplemental Table 1). Importantly, fibronectin and collagen I are direct targets of MT1-MMP,2,3 but MT1-MMP also proteolytically activates other MMPs, such as the gelatinase MMP-2.2,3 We thus repeated the matrix degradation experiments using fluorescently labeled gelatin matrix, with comparable results (Figure 7Q-X and supplemental Table 1). This indicates that also on matrix material that is not a direct proteolytic target of MT1-MMP, kinesin-mediated surface activity of this proteinase is crucial for the regulation of matrix degradation.
Knockdown of kinesin isoforms influences degradation of fibronectin, collagen I, and gelatin matrix. Confocal micrographs of macrophages transfected with siRNA specific for kinesin isoforms and seeded on rhodamine-labeled matrix: fibronectin (A-G), collagen I (I-O), or gelatin (Q-W). (A,I,Q) control siRNA, (B,J,R) KIF5B siRNA, (C,K,S) KIF3A siRNA, (D,L,T), KIF3B siRNA, (E,M,U) double knockdown using KIF5B siRNA and KIF3B siRNA, (F,N,V) KIF1C siRNA, and (G,O,W) MT1-MMP siRNA. Matrix degradation is visible as dark areas by concomitant loss of the fluorescent label. Insets show respective F-actin staining by Cy5-labeled phalloidin. White bar indicates 10 μm. (H,P,X) Statistical evaluation of matrix degradation in cells treated with various siRNAs. The degree of matrix degradation was analyzed by fluorescence measurements of each time 3 × 30 cells. Complete absence of labeled matrix beneath the podosome-covered area of cells was set as 100% degradation. Cells were scored into groups according to the degree of matrix degradation (0%-25%; 26%-100%). *P < .05, **P < .01 compared with control. For specific values, see supplemental Table 1. Bars indicate mean values ± SD.
Knockdown of kinesin isoforms influences degradation of fibronectin, collagen I, and gelatin matrix. Confocal micrographs of macrophages transfected with siRNA specific for kinesin isoforms and seeded on rhodamine-labeled matrix: fibronectin (A-G), collagen I (I-O), or gelatin (Q-W). (A,I,Q) control siRNA, (B,J,R) KIF5B siRNA, (C,K,S) KIF3A siRNA, (D,L,T), KIF3B siRNA, (E,M,U) double knockdown using KIF5B siRNA and KIF3B siRNA, (F,N,V) KIF1C siRNA, and (G,O,W) MT1-MMP siRNA. Matrix degradation is visible as dark areas by concomitant loss of the fluorescent label. Insets show respective F-actin staining by Cy5-labeled phalloidin. White bar indicates 10 μm. (H,P,X) Statistical evaluation of matrix degradation in cells treated with various siRNAs. The degree of matrix degradation was analyzed by fluorescence measurements of each time 3 × 30 cells. Complete absence of labeled matrix beneath the podosome-covered area of cells was set as 100% degradation. Cells were scored into groups according to the degree of matrix degradation (0%-25%; 26%-100%). *P < .05, **P < .01 compared with control. For specific values, see supplemental Table 1. Bars indicate mean values ± SD.
Discussion
MMPs play crucial roles in a plethora of clinically relevant processes, in particular those involving cell migration and invasion of lymphocytes and cancer cells. Regulation of MMP activity is thus, in principle, a promising therapy measure. However, general inhibiton of MMP activity in tissues of patients has elicited side effects such as severe joint pains37 and was thus discontinued. In addition to being unspecific, this approach also targets MMPs at a late stage, that is, after secretion or exposure on the cell surface. For the development of specific and early approaches, it is therefore mandatory to elucidate the intracellular regulation of MMPs, in particular the pathways controlling transport to the cell surface.
Here, we focus on the mechanisms regulating intracellular trafficking and cell-surface exposure of MT1-MMP, a “master switch” protease, which also regulates the activity of several other MMPs.3,7 We show that in primary human macrophages, MT1-MMP, in both mCherry-tagged and endogenous forms, is present in a central accumulation, which surrounds the MTOC and partially overlaps with both cis- and trans-Golgi compartments, as indicated by the respective markers GM130 and TGN46. Moreover, MT1-MMP is enriched at the cell periphery and especially at the leading edge of migrating macrophages. These findings are in good keeping with earlier results showing partial colocalization of MT1-MMP with the MTOC in human fibrosarcoma cells,17 and with TGN46 in human melanoma cells.11
Earlier data also showed a close association of MT1-MMP with microtubules in fixed specimens.17 Using time-lapse video microscopy, we now expand on these findings and show that MT1-MMP positive vesicles actively travel along microtubules. This movement was found to be bidirectional, and individual MT1-MMP vesicles were able to spontaneously switch direction, suggesting the presence of both antero- and retrograde motor proteins on the same vesicle, which is in line with current models.38 Velocity measurements of MT1-MMP vesicles showed that both antero- and retrograde movement along microtubules proceeds with comparable speeds of approximately 0.8 μm/s. These velocities are comparable with reported measurements of movement driven by kinesin (KIF5B: 0.8 μm/s; KIF3A/KIF3B: 0.5-0.6 μm/s)39,40 or cytoplasmic dynein (0.4 μm/s).41 In addition to the long-range transport along microtubules, MT1-MMP–mCherry vesicles also displayed short range transport within the cell periphery. This involved vesicle movement along actin cables and proceeded with lower velocities of approximately 0.4 μm/s, suggesting the involvement of actin-based motors such as myoVa (0.25-0.35 μm/s; Watanabe et al42 ) or myosin VI (0.15 μm/s).43
Ultimately, microtubule- and kinesin-dependent transport is necessary for the exposure of MT1-MMP on the macrophage surface. Use of kinesin constructs with mutations in the P-loop sequence31 pointed to a respective role for KIF5B and KIF3A/KIF3B kinesins, which was confirmed by siRNA-induced knockdown. A combined knockdown of both KIF5B and KIF3B showed a slight additive, although not statistically significant, effect. These results indicate that (1) both KIF5B and KIF3A/KIF3B are involved in MT1-MMP surface exposure, and (2) they function in the same pathway. The remaining surface-associated pool of MT1-MMP–mCherry observed under conditions of KIF5B or KIF3A knockdown indicates that also other factors might influence this process, and particularly the involvement of additional kinesins cannot be ruled out. Alternatively, surface-associated MT1-MMP may represent a pool that is recycled at the cell periphery3 and is not dependent on long-range, microtubule-based transport by kinesins.
However, although kinesins are clearly of major importance for the surface exposure of MT1-MMP, we also find a minor, but significant role of dynein in the regulation of surface MT1-MMP and associated function such as matrix degradation, CD44 and syndecan-1 shedding (supplemental Figure 12). This seems to indicate that, to a certain degree, microtubule-based retrograde transport is involved in maintaining the surface-associated pool of MT1-MMP, possibly through recycling of MT1-MMP at the cell periphery.
Our data also demonstrate, that, through regulating the amount of surface-accessible MT1-MMP, kinesins are also able to alter the percicellular environment of macrophages. This is strikingly exemplified by the reduced shedding of the matrix receptors CD44 and syndecan-1 under conditions of kinesin knockdown. MT1-MMP–dependent shedding of CD44 plays an important role in lymphocyte physiology, for example during the regulation of T-cell adhesion and transendothelial migration.4 Likewise, proteolytic release of the syndecan-1 ectodomain marks the switch to an invasive cell phenotype.9 Kinesin-dependent transport as a basal regulatory mechanism of pericellular MT1-MMP activity is thus likely to be also involved in modulating these phenomena. Moreover, as MT1-MMP also cleaves a wide variety of other matrix receptors such as aggrecan and perlecan, or other surface-associated proteins such as betaglycan and transglutaminase,3 the impact of kinesins on the modification of the macrophage surface proteome is likely to be even more widespread.
In addition to cell-surface proteins, MT1-MMP also cleaves extracellular components such as matrix molecules. Accordingly, we can demonstrate here that kinesin-dependent transport of MT1-MMP also impacts on the matrix degrading ability of macrophages. In macrophages and other monocytic cells, podosomes are the main sites of matrix degradation, and podosome-like structures have been implicated in transcellular diapedesis by lymphocytes44 and in 3-dimensional migration and matrix degradation by macrophages.45 Notably, MT1-MMP has been localized to both podosomes21,46 and the related invadopodia47 and plays a central role in local matrix degradation at these structures.20,48 However, the pathways leading to enrichment of MMPs at podosomes and invadopodia are not well understood. Parts of the machinery for vesicle docking and fusion, such as the v-SNARE VAMP733 or the exocyst complex34 are required for MT1-MMP accumulation at invadopodia. Moreover, a role for both Golgi-derived vesicles11,22 and the microtubule system20 has been speculated upon, but has remained unproven so far. Our findings of podosomes being contacted by Golgi-derived, PIP2-positive MT1-MMP vesicles, which travel along microtubules, thus provide welcome and detailed confirmation of these earlier data and hypotheses.
Moreover, we could show that the kinesins KIF5B and KIF3A/KIF3B are crucially involved in MT1-MMP trafficking as well as MT1-MMP–dependent matrix degradation at podosomes. The latter process is also influenced by KIF1C, a kinesin shown earlier to be important for the dynamics of the podosome structure itself.24 Interestingly, regulation of podosome dynamics is not influenced by several other kinesins, including KIF5B.24 Considering that (1) KIF5B and KIF3A/KIF3B, but not KIF1C, are necessary for the transport of MT1-MMP to the dorsal surface, and (2) MT1-MMP vesicles partially colocalize with KIF5B and KIF3A/KIF3B, but not with KIF1C, KIF5B and KIF3A/KIF3B, but not KIF1C, constitute the basic transport machinery for MT1-MMP to the cell periphery. However, in addition to the delivery of MT1-MMP vesicles to podosomes, proper MT1-MMP activity at these sites also needs additional regulation of the podosome structure through KIF1C.
It is currently unclear how MT1-MMP is delivered to its ultimate substructural site of activity at podosomes. For carcinoma cell invadopodia, it has been shown that recruitment of MT1-MMP involves part of the actin cytoskeleton such as cortactin.49 This could be reflected by contact of MT1-MMP vesicles with the actin-rich core structure of podosomes, as observed in this study. However, we also noted extensive movement of MT1-MMP vesicles along the podosomal ring structure, pointing to a potential involvement of both podosome substructures in the recruitment of MT1-MMP. Final localization of MT1-MMP at the plasma membrane may depend on matrix receptors such as integrins and CD44, which are both found at podosomes,50,51 and are known to interact with MT1-MMP. It is also possible that multiple interaction partners for MT1-MMP are present at podosomes, and only integration of these interactions could determine the final localization of this master switch protease, whose local enrichment is likely to be tightly controlled. The working mode of MT1-MMP at podosomes may be complex, as it proteolytically activates other MMPs, most notably the gelatinase MMP2,2,7 which also localizes to podosomes.52 MT1-MMP may thus regulate matrix degradation through both direct cleavage and pro-MMP2 activation. Our results of MT1-MMP being central for matrix degradation on both fibronectin and gelatin matrices appear to be in line with these thoughts.
In sum, our results show that, in primary human macrophages, MT1-MMP vesicles originate from a Golgi/MTOC compartment and use the microtubule system for long-range intracellular transport. Anterograde movement along microtubules is powered by KIF5B and KIF3A/KIF3B kinesins, while retrograde movement is controlled by dynein. Kinesin-dependent trafficking is necessary for delivery of MT1-MMP to the cell periphery and for its subsequent exposure on the cell surface. The latter is, in part, also dependent on dynein activity and may reflect the involvement of this motor in MT1-MMP recycling.
Accordingly, transport by the identified kinesin isoforms also impacts on surface-associated functions of MT1-MMP such as shedding of CD44 and syndecan-1 or matrix degradation at podosomes. Through regulating the surface-associated pool of MT1-MMP, KIF5B and KIF3A/KIF3B kinesins thus impact on both matrix degradation and the proteolytic modification of matrix receptors. These roles place the identified kinesins in the center stage of macrophage migration and invasion. Kinesin-powered delivery of MT1-MMP may thus turn out to be a useful and early target for the modulation of MT1-MMP–associated cell invasion in macrophages and other lymphocytes. Moreover, as the regulation of MT1-MMP surface activity also plays crucial roles in cancer cell invasion,1,3 this might also be extended to other clinically relevant cell types.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tetsu Akiyama for KIF3A-GFP and KIF3B-GFP, Philippe Chavrier for MT1-MMP–mCherry, Peter Gierschik for PLC-δ1-PH-GFP, Antonella de Matteis for the anti-GM130 antibody, Regine Nowak and Melanie Lachmann for help with flow cytometry, Michelle Peckham for Kif5c-GFP, Thomas Rudel for siRNA testing, Michael Sixt and Roland Wedlich-Söldner for Lifeact-GFP, Richard Vallee for dynamitin-GFP, Wolfgang Ziegler for talin1C-GFP, Barbara Böhlig and Jens Cornils for expert technical assistance, and Martin Aepfelbacher and Peter C. Weber for continuous support. This work is part of the doctoral thesis of C.W.
Work in S.L.'s laboratory is supported by Wilhelm Sander-Stiftung (2007.020.02), Deutsche Forschungsgemeinschaft (LI925/2-1), and the European FP7 program (Marie Curie Actions, T3Net).
Authorship
Contribution: C.W. and S.L. designed experiments; C.W. and M.H. performed experiments; J.F. prepared the anti-mCherry antibody; F.B. prepared human monocytes; and S.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Linder, Institute for Medical Microbiology, Virology and Hygiene, University Medical Center Eppendorf, Martinistr 52, 20246 Hamburg, Germany; e-mail: s.linder@uke.de.